Fig. 1.
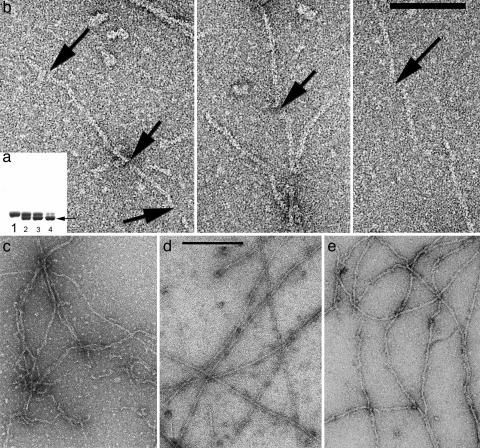
Under conditions where almost all actin is polymerized into filaments, intramolecular disulfide bonds can be introduced into actin subunits containing a triple mutation (L180C/L269C/C374A). These intramolecular disulfides lock the hydrophobic plug of actin (residues 263–274) to the body of the subunit and prevent it from forming a bond with subunits in the opposing strand. (a) SDS/PAGE can show this disulfide formation because the cross-linked monomer runs with higher mobility (lower band, arrow) than the un-cross-linked molecule. Lane 1 is the control, under reducing conditions. After 1 min of oxidation (lane 2), almost half the actin molecules have an intramolecular disulfide. Lanes 3 and 4 show actin after 2 and 15 min of oxidation, respectively. (b) Electron micrographs show the fragmentation of filaments that occurs after disulfide bonds are introduced into filaments that have been first adsorbed to an EM grid. The arrows indicate the breaks that can be seen within filaments. (Scale bar: 1,000 Å.) (c–e) Copolymers of TMR-labeled actin and unlabeled actin (c) are typically shorter than control actin filaments (d), and many kinks and sharp bends are present. Filaments that are examined 2 min after the initiation of polymerization (e) have a much less uniform appearance than the control filaments (d) that are examined 2 h after the initiation of polymerization.