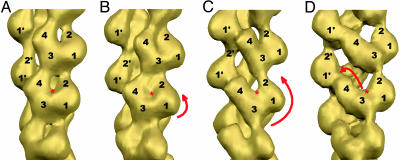
Fig. 2.
Four structural states of F-actin were isolated from complexes of F-actin with either cofilin (32) or Abl-related gene (Arg) kinase. The subdomains (SDs) of actin are numbered, and the nucleotide-binding cleft is marked with a red asterisk. The difference between “regular” state (A) and “intermediate” state (B) is a slight rotation of the whole protomer (short red arrow) that might still allow actin to maintain similar protomer–protomer contacts. The larger rotation of protomers (long red arrow), which we call “tilted state I” (C), does not allow SD2 to interact with SD1 and establishes a new contact between SD2 and SD3 of adjacent protomers in the same long-pitch helix strand. Propeller rotation of SD4 toward the opposite strand (shown by red arrow in D) is called “tilted state II.” In this state, SD4 establishes a contact with SD1 from a protomer on the opposite strand, and this contact is absent in the three other states.