Candida albicans is an important human fungal pathogen. An understanding of fungal virulence factors has been slow because C. albicans is genetically intractable. The recent development of CRISPR/Cas in C. albicans (V. K. Vyas, M. I. Barrasa, G. R. Fink, Sci Adv 1:e1500248, 2015, https://doi.org/10.1126/sciadv.1500248) has the potential to circumvent this problem. However, as has been found in other organisms, CRISPR/Cas mutagenesis efficiency can be frustratingly variable. Here, we systematically examined parameters hypothesized to alter sgRNA intracellular levels in order to optimize CRISPR/Cas in C. albicans. Our most important conclusion is that increased sgRNA expression and maturation dramatically improve efficiency of CRISPR/Cas mutagenesis in C. albicans by ~10-fold. Thus, we anticipate that the modifications described here will further advance the application of CRISPR/Cas for genome editing in C. albicans.
KEYWORDS: CRISPR, Candida albicans, RFP, double-strand-break repair
ABSTRACT
The clustered regularly interspaced short palindromic repeat system with CRISPR-associated protein 9 nuclease (CRISPR/Cas9) has emerged as a versatile tool for genome editing in Candida albicans. Mounting evidence from other model systems suggests that the intracellular levels of single guide RNA (sgRNA) limit the efficiency of Cas9-dependent DNA cleavage. Here, we tested this idea and describe a new means of sgRNA delivery that improves previously described methods by ~10-fold. The efficiency of Cas9/sgRNA-dependent cleavage and repair of a single-copy yeast enhanced monomeric red fluorescent protein (RFP) gene was measured as a function of various parameters that are hypothesized to affect sgRNA accumulation, including transcriptional and posttranscriptional processing. We analyzed different promoters (SNR52, ADH1, and tRNA), as well as different posttranscriptional RNA processing schemes that serve to generate or stabilize mature sgRNA with precise 5′ and 3′ ends. We compared the effects of flanking sgRNA with self-cleaving ribozymes or by tRNA, which is processed by endogenous RNases. These studies demonstrated that sgRNA flanked by a 5′ tRNA and transcribed by a strong RNA polymerase II ADH1 promoter increased Cas9-dependent RFP mutations by 10-fold. Examination of double-strand-break (DSB) repair in strains hemizygous for RFP demonstrated that both homology-directed and nonhomologous end-joining pathways were used to repair breaks. Together, these results support the model that gRNA expression can be rate limiting for efficient CRISPR/Cas mutagenesis in C. albicans.
IMPORTANCE Candida albicans is an important human fungal pathogen. An understanding of fungal virulence factors has been slow because C. albicans is genetically intractable. The recent development of CRISPR/Cas in C. albicans (V. K. Vyas, M. I. Barrasa, G. R. Fink, Sci Adv 1:e1500248, 2015, https://doi.org/10.1126/sciadv.1500248) has the potential to circumvent this problem. However, as has been found in other organisms, CRISPR/Cas mutagenesis efficiency can be frustratingly variable. Here, we systematically examined parameters hypothesized to alter sgRNA intracellular levels in order to optimize CRISPR/Cas in C. albicans. Our most important conclusion is that increased sgRNA expression and maturation dramatically improve efficiency of CRISPR/Cas mutagenesis in C. albicans by ~10-fold. Thus, we anticipate that the modifications described here will further advance the application of CRISPR/Cas for genome editing in C. albicans.
INTRODUCTION
Candida albicans is an opportunistic fungus that is a major cause of fatal infections, especially in individuals with compromised immune systems. Despite its importance as a human pathogen, a deeper understanding of pathogenesis has been limited by the genetic intractability of C. albicans; C. albicans has a parasexual life cycle with no meiosis, and therefore genetic crosses are not possible. Since C. albicans normally exists as a diploid, recessive mutations must be made homozygous. The development of the clustered regularly interspaced short palindromic repeat system with CRISPR-associated protein (CRISPR/Cas) in C. albicans (1) is an important advance that promises to accelerate the pace of progress in Candida albicans biology research.
CRISPR/Cas is a system that provides adaptive immunity in bacteria (2–4). Adaptation of type II CRISPR/Cas for eukaryotic gene editing requires RNA-guided recruitment of the Cas9 nuclease to specific DNA sequences that are adjacent to an “NGG” protospacer adjacent motif (PAM) (5–7). Cas9 is recruited to the target site by a single guide RNA (sgRNA) comprised of a 20-nucleotide guide RNA (gRNA), which directs cleavage specificity by base-pairing complementarity to the target site, and an ~80 nucleotide trans-activating CRISPR-targeting RNA (tracrRNA), which binds Cas9. The sgRNA/Cas9 ribonucleoprotein is recruited to the target site, where Cas9 creates a double-strand break (DSB) 3 bases upstream of the PAM site (3). Since chromosomal breaks are lethal, there is a strong selection for their repair, either by nonhomologous end joining (NHEJ) or by homology-directed repair. The introduction of a DSB can increase the rate of recombination by repair with linear homologous DNA by several thousand-fold (5, 8, 9). Thus, donor repair fragments containing homology to regions flanking the DSB can be designed to introduce deletions or other alterations with single-base-pair precision.
Evidence has supported the idea that intracellular levels of correctly folded, nuclear localized sgRNA limit the rate of Cas9-dependent cleavage (10, 11). Up until now, C. albicans CRISPR/Cas studies have used the RNA polymerase III (Pol III) SNR52 promoter (PSNR52) to drive sgRNA expression (1, 12). Unlike most genes transcribed by Pol III that contain internal promoters within the transcribed region, SNR52 has an upstream promoter (13). Thus, PSNR52 Pol III transcripts are initiated downstream of the promoter but are not confounded by RNA polymerase II (Pol II)-associated 5′ cap and 3′ poly(A) additions that are predicted to influence sgRNA specificity and nuclear retention. PSNR52 has been used successfully for sgRNA expression in both Saccharomyces cerevisiae and C. albicans (1, 14), but its relative efficiency in C. albicans has not been compared to those of other sgRNA delivery schemes.
Our attempts to use the PSNR52 CRISPR system described by Vyas et al. (1) did not lead to high-efficiency mutagenesis of several C. albicans target genes, despite the use of different gRNAs that target different sites within each gene. Therefore, to improve CRISPR mutagenesis, we sought to optimize sgRNA expression and CRISPR/Cas efficiency in C. albicans by comparing different promoters and RNA processing mechanisms. Our results demonstrate that gRNA expression can indeed be rate limiting for efficient DSB cleavage in C. albicans, and we describe new modifications that can increase the efficiency of gene editing in C. albicans by 10-fold. While our major goal was to optimize gRNA expression, our experiments also revealed that in addition to homology-directed repair, under certain conditions, Cas9-dependent DSB can be repaired by nonhomologous end joining at low frequency.
RESULTS AND DISCUSSION
Assay system.
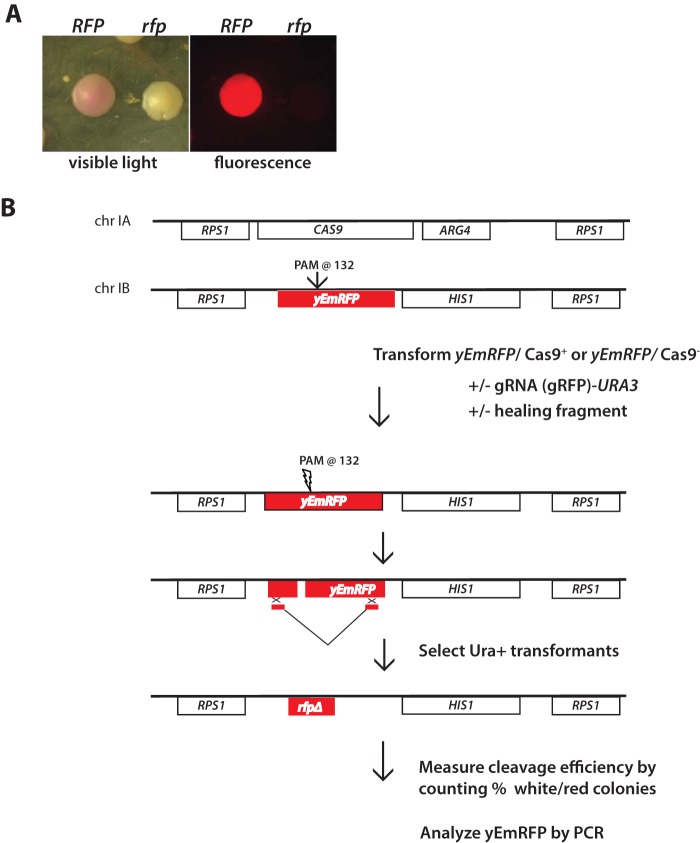
To examine gRNA-dependent Cas9 nuclease activity, the C. albicans gene encoding codon-optimized enhanced monomeric red fluorescent protein (yEmRFP [henceforth referred to as RFP]) was targeted (15). C. albicans strains expressing RFP display a distinct pink colony color phenotype as well as bright red fluorescence (16). Inactivation of RFP by mutation results in a reversal of the pink colony color, red fluorescence phenotype, so colonies derived from rfp mutants could be easily distinguished by their white color and absence of fluorescence (Fig. 1A).
FIG 1 .
CRISPR/Cas targeting of RFP in C. albicans. (A) Yeast colonies that express functional RFP are pink and are fluorescent and can be easily distinguished from colonies that arise through CRISPR-mediated deletion of RFP, which are white and nonfluorescent. Panel B depicts the strategy for quantitating Cas9- and sgRNA-dependent cleavage of RFP. RFP (EPC1) or RFP CaCAS9 (EPC2) hemizygous strains were constructed as described in Materials and Methods. These strains were transformed with or without a donor healing fragment and a series of URA3-marked plasmids that differ in expression of an RFP sgRNA (Fig. 3). The gRNA targets a 20-bp DNA sequence proximal to the RFP PAM site at position 132. The number of white and red colonies in each transformation was counted, and cleavage efficiency was calculated as the percentage of white colonies in the population.
We constructed RFP hemizygous strains in which HIS1-marked RFP was integrated at single copy at the RPS1 locus (Fig. 1B). As a control for Cas9-dependent rfp mutations, we constructed RFP strains that do or do not also express CAS9. C. albicans uses a noncanonical genetic code that reads the leucine CTG codon as serine. Thus, a synthetic Candida-optimized CAS9 gene (CaCAS9) was designed to alter each CTG serine codon to an AT-rich leucine codon. In addition, the codon bias of the entire gene was maximized toward AT-rich codons (see Materials and Methods). These RFP/CaCAS9 strains were transformed with URA3-marked sgRNA plasmids (described below) that targeted RFP cleavage proximal to the PAM site at position +132 of the RFP open reading frame (ORF) (Fig. 1B). The efficiency of Cas9/sgRNA-dependent cleavage was measured by determining the percentage of white, nonfluorescent rfp mutant colonies in the population of transformants.
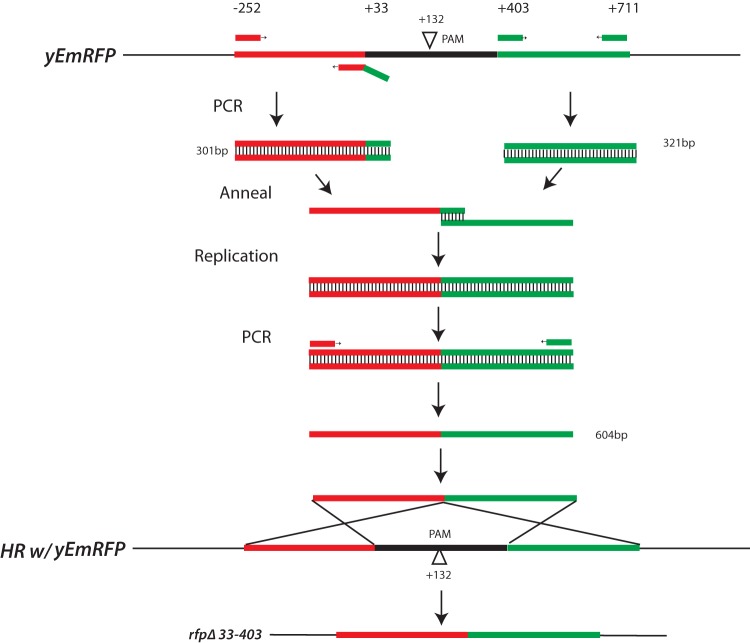
These transformations were performed in the presence or absence of a donor repair fragment designed to delete 370 bp of the RFP ORF after homology-directed repair. This repair fragment was made using overlap extension PCR and contained 285-bp arms of upstream and 308-bp downstream sequence homology to regions flanking the DSB (see Materials and Methods) (Fig. 2). To confirm that RFP in white colonies was correctly deleted by recombination with the repair fragment, RFP was amplified by PCR, analyzed by gel electrophoresis for the presence of the deleted allele, and also sequenced (see below). This screen allowed a simple and accurate way to measure the efficiency of Cas9-dependent cleavage and repair of RFP.
FIG 2 .
Construction of the RFP donor repair fragment. The donor repair fragment used for repair of the Cas9-induced DSB was constructed by overlap PCR. Two fragments were amplified by PCR, one of which contains a 3′ end that is homologous to the 5′ end of the other. After the two fragments are mixed, these homologous regions anneal to one another, and then a round of DNA replication produces a single fragment with arms of homology to the 5′ and 3′ regions of RFP, but which is deleted for 370 bp of the ORF, including the PAM site at position 132. Homologous recombination of this rfpΔ33-403 fragment with chromosomal RFP can easily be detected by PCR amplification (Fig. 4).
sgRNA delivery schemes.
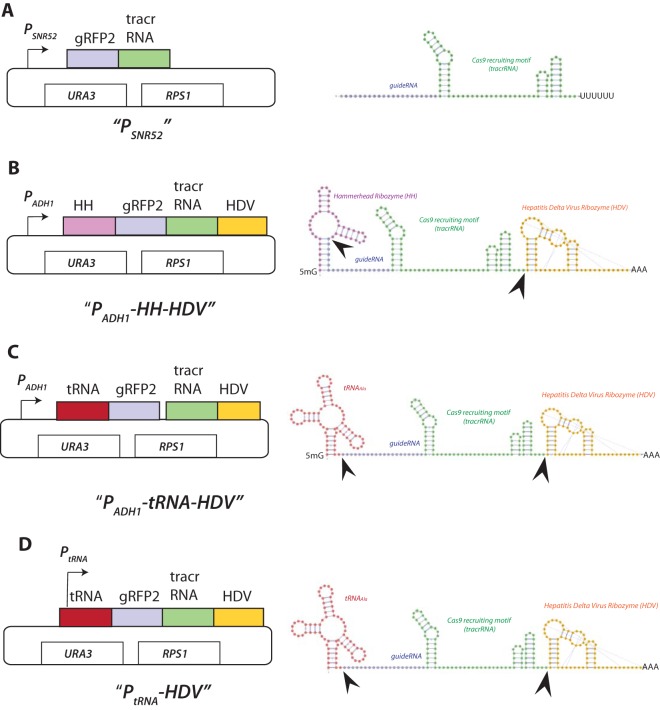
To optimize sgRNA expression, several parameters were varied, including the promoters used to drive transcription, as well as removable flanking sequences designed to be posttranscriptionally cleaved to produce transcripts with precise 5′ and 3′ ends. A schematic diagram of the different constructs and the predicted RNA transcripts is shown in Fig. 3, and their DNA sequences are listed in Fig. S1 in the supplemental material. Each construct had the same sgRNA sequence but differed in the promoters used to drive sgRNA transcription and/or flanking sequences that encode ribozymes or tRNAs that are posttranscriptionally cleaved.
FIG 3 .
Schematic diagram of sgRNA expression cassettes and the structures of their predicted encoded RNA. The backbone of each of these plasmids is based on the URA3 integrating CIP10 plasmid (35) (see Materials and Methods). RNA secondary structure visualization was performed using VARNY (http://varna.lri.fr/) (41). (A) PSNR52 drives transcription of the sgRNA that consists of the 20-nucleotide gRFP (purple) fused to the 85-nucleotide Cas9 recruiting tracrRNA (green). Note that this sgRNA is identical in all four delivery schemes. The gRNA has the requisite 5′ G and a 3′ poly(U) tail. (B) PADH-HH-HDV. The sgRNA is flanked by the 5′ hammerhead (HH [pink]) and 3′ hepatitis delta virus (HDV [yellow]) ribozymes. HH-sgRNA-HDV transcription is driven by the strong ADH1 promoter. The stem structure formed between the 5′ HH and 5′ gRNA forms the structural motif recognized for autocleavage (depicted by arrowhead). The 3′ cleavage relies on the autonomous pseudoknot structure of HDV (depicted by arrowhead). (C) PADH-tRNA(−HDV). This construct is exactly like that shown in panel B, but the hammerhead sequence is replaced by the 75-bp C. albicans alanine tRNA gene. In this scheme, the mature 5′ end of the sgRNA is generated by endogenous RNase Z, which recognizes the stem structure formed by tRNA 5′ and 3′ sequences. 3′ cleavage relies on the autonomous stem-loop structure of HDV (depicted by arrowhead). Transcription of the tRNA-sgRNA-HDV is driven by the strong ADH1 promoter. (D) PtRNA(−HDV). This construct is exactly like that of panel C, but the PADH1 sequence is deleted. In this cassette, RNA Pol III transcription is driven by the internal A and B box elements of the tDNA promoter, and the mature 5′ end of the sgRNA is generated by endogenous RNase Z, while 3′ cleavage relies on the autonomous pseudoknot of HDV (depicted by arrowhead).
pADH/tRNA plasmids used for high-efficiency gRNA expression. (A) Schematic diagram of the CIp-PADH/tRNA vector that contains a cloning cassette containing two nonpalindromic SapI sites, separated by a unique ClaI, at the tRNA-tracrRNA junction. These RPS1 integration plasmids are marked with URA3 (p494) or ura3-dpl200 (p501). (B) The sequence of the SapI cassette (blue) at the tRNA (green)-tracrRNA (purple) junction. The positions of ClaI and SapI sites within the cassette are underlined, and arrows depict the SapI cleavage sites. Beneath the SapI cassette is an example of how to design two gRNA oligonucleotides (boxed, using LEU2 gRNA). Note the gRNA (red) oligonucleotides contain CAA overhangs that create sticky ends when annealed, which allow ligation of these oligonucleotides into SapI-digested vector. Ligation results in recreation of the RNase Z tRNA recognition cleavage site, as well as the correct fusion of the gRNA with the tracrRNA. Correct ligation products are screened by the loss of the ClaI site. Download FIG S1, PDF file, 1.8 MB (1.9MB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The first construct used the Pol III PSNR52 to drive sgRNA expression (PSNR52-sgRNA). The C. albicans PSNR52 promoter sequence and sgRNA junction were the same as those used in previous studies (Fig. 3A) (1). PSNR52 Pol III transcripts are initiated at a purine, G or A, and are terminated by a poly(U) stretch (six in yeast) (13).
The second delivery scheme used the strong Pol II ADH1 promoter (PADH1) to drive sgRNA transcription (Fig. 3B). Because PADH1 is a strong constitutive promoter, we reasoned that PADH1 may increase the levels of sgRNA. To prevent potential interference of sgRNA specificity by the 5′ cap and 3′ poly(A) tail associated with Pol II transcripts, self-cleaving hammerhead (HH) and hepatitis delta virus (HDV) ribozymes were added to the 5′ and 3′ ends of sgRNA. The presence of these ribozymes at the sgRNA ends may also protect it from nucleases and therefore further increase its intracellular levels. In the design of this cassette, the first six nucleotides of the HH ribozyme are complementary to the first six nucleotides of the gRNA, thus allowing the formation of the stem structure required for HH self-cleavage at the gRNA-HH junction (17). The sites of the HH and HDV predicted cleavage sites are indicated by the arrowheads in Fig. 3B.
To compare the efficiency of sgRNA maturation, the third delivery scheme used the same strong PADH1 to transcribe sgRNA, but its 5′ end was flanked with C. albicans tRNAAla instead of the HH ribozyme (Fig. 3C). The rationale for this idea was 2-fold. First, endogenous RNase P and Z mediate the robust posttranscriptional cleavage of 5′ and 3′ ends of pre-tRNAs (18, 19). These endogenous RNase activities may be more efficient than HH autocatalysis and therefore lead to improved sgRNA maturation. Second, cleavage by RNase Z at the tRNA-sgRNA junction requires recognition of sequences that are only within the tRNA. Therefore, this architecture, if efficient, would provide a more portable platform for swapping out the 20-bp gRNA coding sequence with any another gRNA. This is not possible using HH ribozyme, whose unique 5′ sequence is constrained by each different gRNA. The sequence of the sgRNA-tRNA junction was designed to yield the precise sgRNA 5′ end after cleavage with RNase Z (11, 20). The sites of the tRNA and HDV predicted cleavage sites are indicated by the arrowheads in Fig. 3C.
As tRNAs are very abundant, we considered the possibility that the tRNA promoter itself may be efficient in directing sgRNA expression (11, 20). To test this, the fourth delivery scheme used the tRNAAla fused to sgRNA-HDV (Fig. 3D). Cleavage of tRNA at its 3′ trailer by endogenous RNase Z (see arrows for cleavage site) allows maturation of sgRNA with a precise 5′ end. The sites of the tRNA and HDV predicted cleavage sites are indicated by the arrowheads in Fig. 3D.
Measuring RFP mutation as a function of sgRNA delivery.
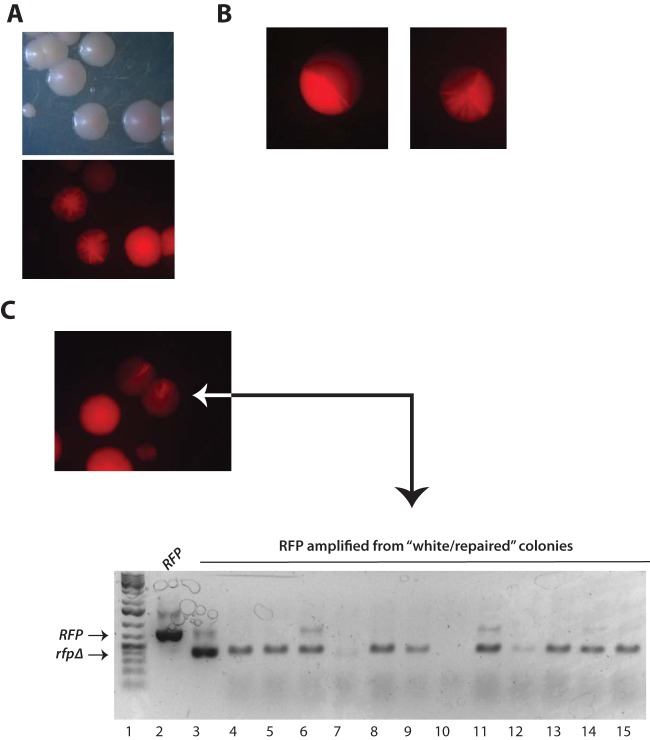
To compare their relative activities, each of the URA3-marked CIp gRNA plasmids described above, or a negative (−) gRNA vector alone (−gRNA) was transformed side by side into RFP strains that did (EPC2) or did not (EPC1) express Cas9 (see Materials and Methods). Each transformation was performed in the presence or absence of a repair fragment (see Materials and Methods). After 2 days, colonies from each transformation were counted by fluorescence microscopy to determine the number of white and red colonies for each transformation. Unexpectedly, in addition to pure red and white colonies, under certain conditions, we observed red colonies with white sectors (Fig. 4). White or white-sectored colonies were seen at a frequency of less than 10−4 in control experiments, in which the isogenic Cas9− EPC1 strain was transformed with gRNA, vector, and/or the donor repair fragment (data not shown) (Table 1). White or white-sectored colonies were also not observed in Cas9+ EPC2 cells transformed with vector lacking gRNA with or without the donor repair fragment. These control experiments demonstrated that the high frequency of white or white-sectored colonies was not due to spontaneous looping out of RFP, which is flanked by RPS1 repeats, or by replacement of RFP with the CIp-gRNA plasmid during integration at RPS1. Instead, white and white-sectored colonies were dependent on the expression of both Cas9 and sgRNA (Fig. 5C; Table 1). Each white sector represented a Cas9-mediated RFP cleavage event that occurred in a cell at the vertex of the white sector. The sizes and numbers of white sectors ranged from those that were 1/2 the colony, in which RFP cleavage likely occurred in one daughter cell of the first division, to those that appear mostly white but had a remaining sliver of red cells in a colony (Fig. 4A and B). The rfp mutation that gave rise to white sectors was stable, since restreaking a sectored colony on –Ura plates resulted in colonies that were almost completely white (not shown). This sectoring phenotype could explain why PCR analysis of RFP amplified from genomic DNA from what appeared to be visually “white/healed” colonies occasionally yielded both full-length and deleted RFP products (Fig. 4C).
FIG 4 .
Sectored colony phenotype of RFP mutants. (A) Comparison of red, white, and sectored colony phenotype in visible and red fluorescent light. (B) Examples of various degrees of sectoring that range from mostly red to mostly white. (C) Red and white phenotypes in a sectored colony are genetically stable. A single sectored colony was restreaked on nonselective medium. Below is shown PCR analysis of full-length and rfpΔ33-403 from white “repaired” colonies isolated after transformation in the presence of a donor repair fragment.
TABLE 1 .
RFP mutagenesis with PADH-tRNA-gRFP-HDV
| Transformationa | Total no. of transformantsb | % of colonies: |
% of transformants: |
|||
|---|---|---|---|---|---|---|
| Red | White | Sectored | His+c | Arg+d | ||
| (−) Cas9 (EPC1), (+) vector (−gRFP) | ~1,000 | 100 | 0 | 0 | 100 | 0 |
| (−) Cas9 (EPC1), (+) gRFP | ~1,000 | 100 | 0 | 0 | 99 | 0 |
| (−) Cas9 (EPC1), (+) gRFP, (+) healing fragment | ~1,000 | 100 | 0 | 0 | 100 | 0 |
| (+) Cas9 (EPC2), (+) vector (−gRFP) | ~1,000 | 100 | 0 | 0 | 100 | 80 |
| (+) Cas9 (EPC2), (+) gRFP | ~1,000 | 8 | 90 | 2 | 30 | 80 |
| (+) Cas9 (EPC2), (+) gRFP, (+) healing fragment | ~1,000 | 8 | 92 | 0 | 97 | 73 |
Transformations were performed with URA3-marked PADH-tRNA-gRFP-HDV plasmid pND482 (+gRFP) or vector lacking gRFP (−gRFP). (−), donor repair fragment absent; (+), donor repair fragment present. The results shown represent an average of 5 separate experiments, in which the percentage of white colonies ranged from 90 to 98%.
Number of uracil prototrophic transformants.
Percentage of uracil prototrophic transformants that were histidine prototrophs.
Percentage of uracil prototrophic transformants that were arginine prototrophs.
FIG 5 .
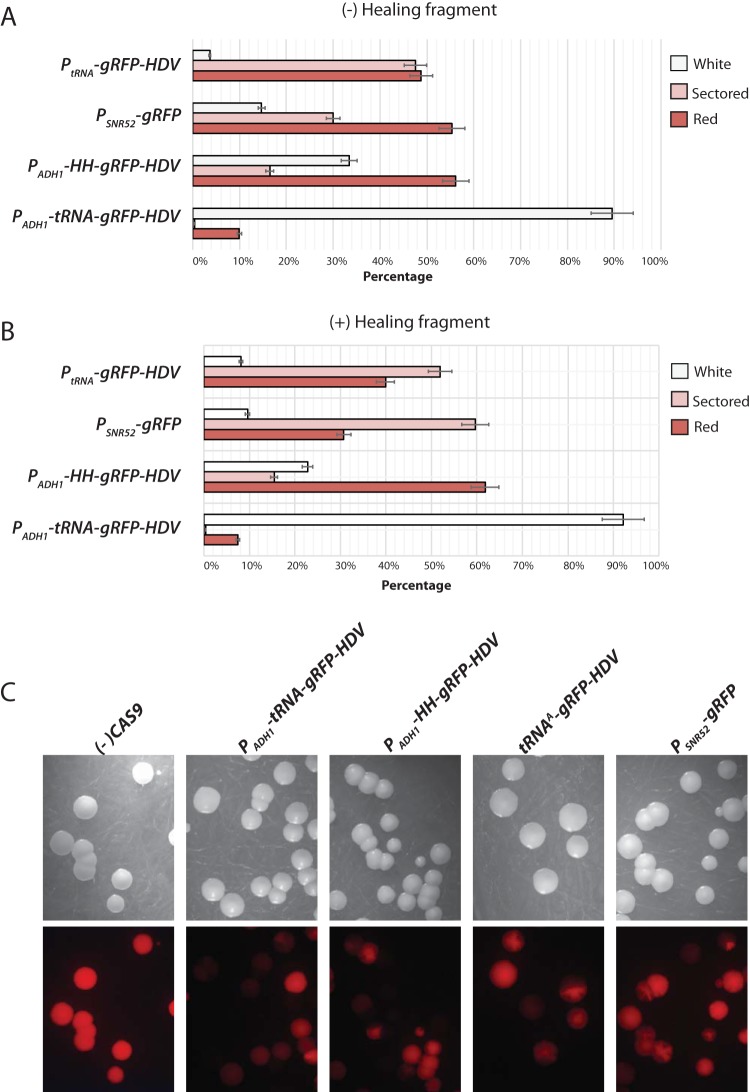
RFP CRISPR mutagenesis as a function of sgRNA delivery. RFP Cas9 strain EPC2 was transformed with each of the plasmids depicted in Fig. 3. Shown are the percentages of white mutant (rfpΔ), red (RFP), and sectored colonies that arose after 2 days at 30°C. Transformations were performed in the presence of a donor homologous repair fragment (A) or in its absence (B). The data represent an average from three separate experiments in which all plasmids (plus or minus the repair fragment) were transformed side by side. Approximately 200 colonies per plate were counted and scored as red, white, or sectored. For each experiment, controls were included in which all plasmids were transformed in the isogenic strain that lacks Cas9 (not shown). (C) Light and red fluorescent images of colony phenotype as a function of different sgRNA delivery plasmids depicted in Fig. 3. Note the near absence of sectored colonies when sgRNA is efficiently delivered (PADH-tRNA-gRFP-HDV) compared to when poorly delivered (PtRNA or PSNR52).
Thus, to more accurately quantitate the relative efficiency of sgRNA delivery, we counted the number of white, red, and sectored colonies produced after transformation with each of the different plasmids, in the presence or absence of healing fragment. These results, shown graphically in Fig. 5A and B, demonstrated a clear hierarchy of cleavage efficiency as measured by the percentage of pure white rfpΔ mutant colonies in the population, in the following order: PADH1-tRNA (~94% white) > PADH-HH (~22% white) > PSNR52 (9% white) = PtRNA (~8% white). When sgRNA transcription was driven by the Pol II ADH1 promoter but flanked by the 5′ hammerhead ribozyme instead of the tRNA, the Cas9-directed mutation frequency was reduced by ~3.5-fold. When sgRNA transcription was driven by the Pol III SNR52 or tRNA promoters, the mutation frequency was reduced by ~10-fold. While the experiments shown in Fig. 5 were performed using CIp-based integration plasmids, the same trend was observed using CaARS-containing plasmids (data not shown). Therefore, we inferred that the high efficiency observed when sgRNA was delivered using the PADH1-tRNA plasmid is due to the combination of (i) increased Pol II PADH1 promoter strength, compared to the Pol III PSNR52 or PtRNA, and (ii) the presence of the tRNA flanking sequence. These results support the idea that the presence of the tRNA may promote sgRNA nuclear retention or stabilization and in addition is processed by endogenous RNase Z more efficiently than HH autocatalysis. An additional factor that may explain the reduced efficiency of transcripts driven by PSNR52 is that the sgRNA is constrained by a 5′ purine, which is the favored nucleotide for transcription initiation by PSNR52. A non-base-paired 5′ G, which is built into the cloning site of PSNR52 CRISPR vectors, may result in 5′ overhang in the sgRNA-target DNA complex that could impact sgRNA efficiency, although this has not been formally tested. Thus, of the sgRNA delivery schemes we tested, the PADH1-tRNA-driven sgRNA expression was the most efficient, by almost 10-fold.
Quantitation of sectored colonies demonstrated a strong correlation between the abundance of sectored colonies and the inefficiency of the sgRNA delivery. In a population of PADH1-tRNA transformants, over 90% of colonies were white, while less than 1% were sectored. Conversely, in a population of PtRNA transformants, less than 10% of colonies were white, while more than 50% were sectored (Fig. 5C). These results are shown quantitatively in Fig. 5. One interpretation of these results is that efficient Cas9-dependent cleavage requires a threshold level of sgRNA that is not met in founder transformants when sgRNA is poorly expressed. After one or more cell divisions, daughter cells may eventually accumulate sufficient levels of sgRNA to allow Cas9 DSB and repair.
RFP DSB repair.
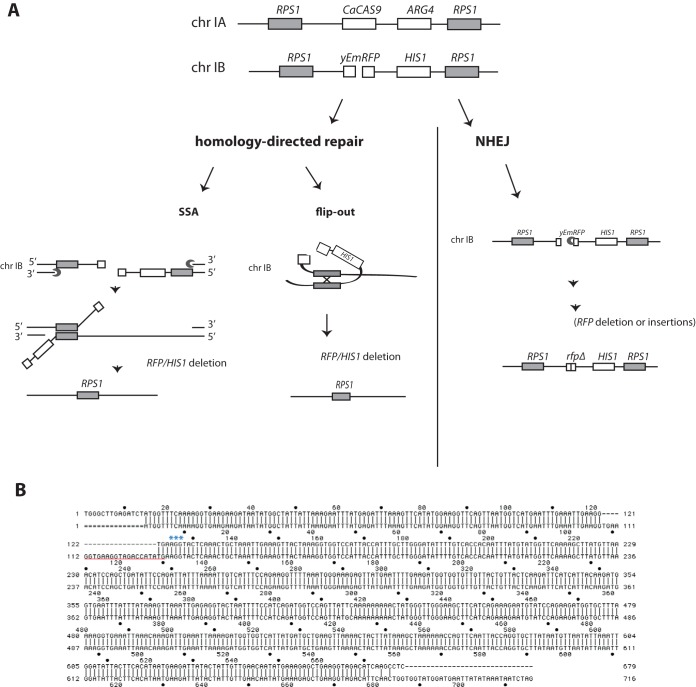
Surprisingly, we observed that the relative numbers of white, red, and sectored colonies for each of the different sgRNA plasmids were similar whether or not a donor repair fragment was included in the transformation (compare Fig. 5A and B). These results raised the question of how breaks in RFP were repaired in the absence of homologous recombination with the repair fragment. The strain used for these experiments is hemizygous for RFP. Since this sequence is not repeated anywhere else in the genome, this DSB cannot be repaired by gene conversion. However, RFP is flanked by a duplication of RPS1 loci (see Materials and Methods) (Fig. 1 and Fig. 6A), and there are additional duplicated RPS1 loci that were introduced by integration of the gRNA plasmid, as well as CAS9. Thus, Cas9-mediated DSB in RFP could stimulate its deletion by a variety of homology-directed pathways that involve recombination between adjacent RPS1 loci (i.e., by flip-out or single-strand annealing as depicted in Fig. 6A) (21) or even RPS1 on the homologous chromosome (Fig. 6A). These types of homology-directed repair pathways would lead to deletion of RFP sequence, which includes RFP and HIS1. An alternative means of DSB repair is nonhomologous end joining (NHEJ), in which the breaks are directly ligated, although this is usually accompanied by short insertions or deletions (Fig. 6A) (22, 23).
FIG 6 .
Models for DSB repair at the hemizygous RFP locus by homology-mediated repair and NHEJ. (A) Schematic diagram of homology-mediated and NHEJ pathways of DSB repair in RFP in the absence of donor repair fragment. A variety of homology-directed repair pathways between duplicated RPS1 loci are possible. Both flip-out and single-strand annealing (SSA) are consistent with the observed simultaneous loss of both RFP and HIS1 when a DSB is introduced in RFP. (B) Alignment of the wild-type RFP sequence with that of RFP amplified from a white colony isolated after CRISPR mutagenesis in the absence of a donor repair fragment. The sequence targeted by the RFP gRNA is underlined in red and precedes the PAM site (AGG, denoted with blue asterisks) at n = 132. Note that the deletion begins 3 bases from the PAM, precisely at the predicted Cas9 cleavage site.
To investigate how these DSB were repaired in the absence of donor healing fragment, RFP from these white colonies (~30) was amplified by PCR, analyzed by gel electrophoresis, and subjected to DNA sequencing. If DSBs at RFP were repaired by homologous recombination between adjacent RPS1 loci, then both the RFP and HIS1 genes should be completely deleted. To test this, these colonies were also replica plated on −His plates to determine the presence or absence of HIS1. The result of this experiment demonstrated that by PCR analysis, 70% of these white colonies lacked the RFP gene entirely and were also histidine auxotrophs, suggesting that in these colonies, RFP DSB repair occurred via a homology-directed repair pathway (Fig. 6A).
The remaining 30% of these white colonies contained RFP of a similar electrophoretic mobility as the full-length gene. As exemplified in Fig. 6B, the alignment of wild-type RFP with one such mutant showed the presence of a 21-bp deletion that removes the PAM site and sgRNA targeting sequence (Fig. 6B [GGTGAAGGTAGACCATATGAA represents the gRNA sequence underlined in red]). The mutant DNA sequence (shown in Fig. 6C and Fig. S2 in the supplemental material) indicates that the Cas9 cut site occurred 3 bases from the PAM (AGG at position 132, depicted by blue asterisks in Fig. 6B). This result suggested that repair occurred via nonhomologous end joining, resulting in a short deletion. This was a surprising result because several studies have demonstrated that, in C. albicans as in S. cerevisiae, homologous recombination is a vastly preferred mechanism for repair of DSB since mutations that knock out homologous recombination affect the sensitivity of strains to agents that produce DSB, while mutations in NHEJ do not (24, 25). Our results suggested that although homology-directed repair is the predominant pathway, DSBs can be repaired in C. albicans by NHEJ.
NHEJ DNA sequence trace. Shown is a partial DNA trace of an rfp mutant allele whose sequence is consistent with an NHEJ event. A schematic diagram of a portion of the sequence aligned to RFP is depicted, showing deletion endpoints. (See the sequence alignment in Fig. 6B.) Download FIG S2, PDF file, 0.2 MB (184.4KB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Extension of system to endogenous genes.
The results described above demonstrated a system for efficient targeting and repair of RFP. However, RFP was present in the chromosome as single copy and flanked by direct repeats, which may affect its mutability. Therefore, to confirm the utility of the PADH1-tRNA sgRNA delivery scheme for deletion of endogenous diploid genes, we constructed URA3- or recyclable ura3-dpl200 (26)-marked PADH-tRNA vectors (pND494 and pND501) to allow optimized expression of any gRNA. These plasmids allow ligation of short (23-bp) annealed oligonucleotides into a cloning cassette containing two nonpalindromic SapI sites at the tRNA-gRNA junction (see Materials and Methods and Fig. S1). This cassette was designed to maintain the RNase Z cleavage recognition site at the tRNA-gRNA junction as well as the seamless fusion of gRNA to tracrRNA after ligation of annealed gRNA oligonucleotides (Fig. S1).
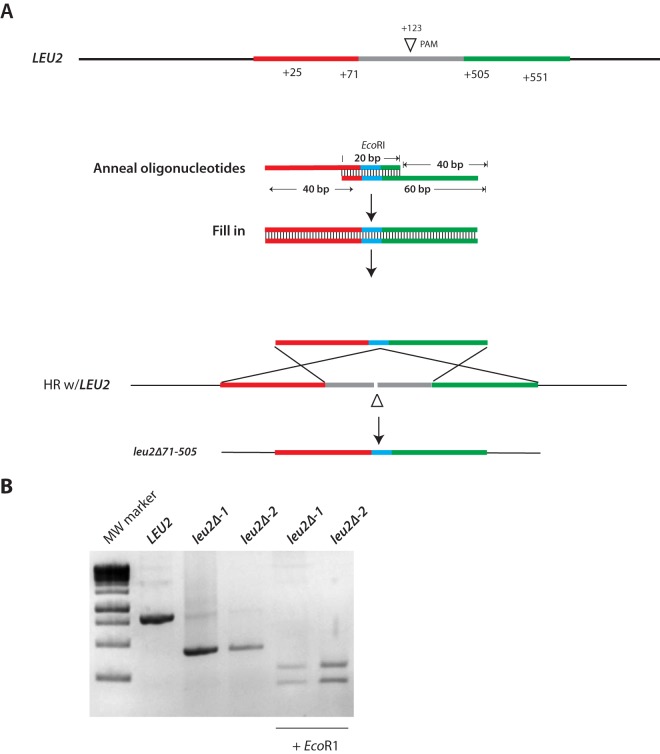
As proof of principle, we targeted LEU2 with a gRNA complementary to the region proximal to the PAM at position +123 of the LEU2 ORF (Fig. 7). A Cas9+ strain (HNY30) was transformed with a LEU2-specific gRNA plasmid marked with URA3 or vector lacking gLEU2 (Table 2). These transformations were performed in the absence or presence of a donor repair fragment designed to replace a 434-bp fragment within the LEU2 ORF with a unique EcoRI site (see Materials and Methods) (Fig. 7A). This repair fragment contained 47-bp arms of homology to regions flanking the break. As noted by Vyas et al. (1), we found that repair fragments with arms of longer homology (80, 250, or 500 bp) did not improve the efficiency of mutagenesis (not shown). Controls included the same transformations but of a Cas9− isogenic strain (BWP17). To quantify mutations in LEU2, Ura+ transformants were patched on plates containing synthetic dropout medium without uracil [SD(−Ura)] and then replica plated on SD(−Ura) and SD(−Leu) to identify leucine auxotrophs (Table 2). LEU2 from both Ura+ and Leu− transformants was genotyped by PCR (Fig. 7B; Table 2).
FIG 7 .
Markerless deletion of LEU2. (A) Schematic diagram of the LEU2 locus, showing the PAM site at position 123. Sequences homologous to the repair fragment are colored in green and red. Each 60-mer oligonucleotide ends with a 20-bp sequence of complementarity, including a restriction site that is absent in LEU2 (EcoRI), shown in blue. When annealed and extended, this repair fragment contains 47 bp of homology to sequence flanking the DSB. Homologous recombination (HR) results in a 434-bp deletion of LEU2, which is replaced by a unique EcoRI site. (B) PCR analysis of DNA from LEU2 and leu2Δ mutants isolated by CRISPR (see text). EcoRI digestion of PCR products verified the leu2 deletion genotype. MW, molecular weight.
TABLE 2 .
LEU2 mutagenesis
| Transformationa | No. of transformants: |
% of: |
||
|---|---|---|---|---|
| Ura+ | Leu−b | LEU2/leu2 heterozygotesc | leu2Δ/leu2Δ homozygotesd | |
| (−) Cas9, (+) vector | 620 | 0 | 0 | 0 |
| (−) Cas9, (+) gLEU2 | 700 | 0 | 0 | 0 |
| (−) Cas9, (+) gLEU2, (+) repair fragment | 650 | 0 | 0 | 0 |
| (+) Cas9, (+) gLEU2 | 28 | 0 | 0 | 0 |
| (+) Cas9, (+) gLEU2, (+) repair fragment | 760 | 608 | 0 | 80 |
Transformations were performed with BWP17 (Cas9−) or HNY30 (Cas9+) with URA3-marked LEU2 gRNA plasmid, p499 (+gLEU2), or the vector that lacks gRNA (p494). (−), donor repair fragment absent; (+), donor repair fragment present. The results shown represent the average from 3 experiments.
The number of Ura+ transformants that were leucine auxotrophs.
The percentage of LEU2/leu2Δ heterozygotes was based on PCR analysis of LEU2 using genomic DNA isolated from 40 random Ura+/Leu+ transformants.
The percentage of leu2Δ/leu2Δ homozygotes was based on PCR analysis of LEU2 amplified from genomic DNA isolated from 60 random Ura+/Leu− transformants.
The results of these experiments revealed some important differences between LEU2 and RFP mutagenesis. First, in contrast to what was observed with RFP, the number of Ura+ gLEU2 transformants that were obtained in the absence of healing fragment was ~25-fold lower than in its presence. This reduction in transformation was dependent on the presence of both Cas9 and LEU2-specific gRNA, suggesting it was caused by DSBs (Table 2). Second, in the absence of healing fragment, none of the viable Ura+ transformants were leucine auxotrophs (Table 2). Thus, a plausible explanation for this reduction is that donor repair fragment is required to repair breaks in LEU2, and that unrepaired breaks are lethal. This implies that other non-homology-directed repair pathways, including NHEJ, occur very rarely. Third, in the presence of healing fragment, ~80% of Ura+ transformants were Leu−. PCR analyses of these Leu− mutants suggest that all of them arose through homologous recombination with the donor repair fragment (Table 2; Fig. 7B). Finally, from random PCR analysis of Ura3+ transformants, we found no evidence for heterozygosity, since PCR amplification products from single colonies yielded either wild-type or truncated (EcoRI-sensitive) LEU2, but not both (Table 2; data not shown). The lack of heterozygosity was not specific for LEU2 since similar results were obtained for other nonessential endogenous genes that were targeted for mutagenesis (data not shown), including ANP1, VAN1, and BMT1, whose further characterization will be described elsewhere.
These experiments demonstrate the applicability of this expression system for creating DSBs in endogenous diploid genes with high efficiency. This system has several advantages compared to others that have been described (1, 27). First, the higher mutation frequency, through use of the ADH1 promoter and tRNA instead of the SNR52 Pol III promoter, means that fewer yeast colonies require screening in order to identify a desired mutation. Second, while the transient system described by Min et al. (12) is very simple, we found that even under optimal gRNA expression, the mutation frequency is too low for mutagenesis without selection of a marker-linked donor repair fragment. The expression system described in the present study facilitates “scarless” mutations, although it requires selection of marker-linked gRNA. gRNA can be linked to URA3 as described here or nourseothricin resistance as described by Vyas et al. (1). The advantage of uracil selection is that it is faster and cheaper than Natr. Also, certain mutations—for instance those that strongly interfere with cell wall biosynthesis—display altered nourseothricin sensitivities (our unpublished observation), which can complicate their isolation using Natr selection. On the other hand, recycling ura-dpl200 requires counterselection with 5-fluoroorotic acid (FOA), which may bias toward selection of aneuploidy (28). A basic protocol for the day-to-day timeline for a single-gene knockout using the vectors described in this study is outlined in Fig. S3 in the supplemental material.
Timeline for CRISPR/Cas mutagenesis in C. albicans. Download FIG S3, PDF file, 0.1 MB (80.4KB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Concluding remarks.
Genetic analysis of C. albicans has been complicated because it is a diploid that does not readily undergo sexual reproduction. Without CRISPR, genetic modifications, including knockouts, must be applied to both chromosomes, requiring sequential modification of each locus. The application of the CRISPR/Cas system as described by Vyas et al. (1) has enormous potential because it can circumvent these problems. However, as has been found in other systems, the efficiency of CRISPR/Cas can be frustratingly variable. Studies from other systems suggest that several factors influence efficacy of CRISPR/Cas, including (i) location and accessibility of gRNA target site, (ii) gRNA sequence, and (iii) sgRNA intracellular levels. Here, we systematically examined parameters hypothesized to alter sgRNA intracellular levels in order to optimize CRISPR/Cas in C. albicans. Our most important conclusion is that increased sgRNA expression and maturation dramatically improve efficiency of CRISPR/Cas mutagenesis in C. albicans. Large-scale analyses of the sgRNA target site effects, chromatin structure, and gRNA sequence preferences have led to an increasing knowledge base, as well as online tools that help design gRNAs. Features of a “good” gRNA include guanines at the −1 and −2 positions (i.e., a 3′ GG) and cytosine at the −3 DNA cleavage site and at +1 relative to the N0G0G0 PAM site (29–32). Features of a good target position appear to be nucleosome-free locations upstream of transcriptional start sites (29–31, 33, 34). It is notable that neither the gRFP nor its target location used in the present study conforms to any of these predictive guides, yet when optimized for expression, it nevertheless resulted in an almost 100% mutation frequency. These results suggest that in C. albicans, sgRNA levels may in part compensate for a less than optimal gRNA design. Thus, we anticipate that the modifications described here will further advance the application of CRISPR/Cas for genome editing in C. albicans.
MATERIALS AND METHODS
Plasmid construction.
The plasmids and their relevant features are listed in Table 3, and the sequence of each relevant cassette and the oligonucleotides used for the construction are listed in Table S1 in the supplemental material. All DNA generated by PCR was verified by DNA sequence analysis.
TABLE 3 .
Plasmids used in this study
| Plasmid no. | Name | Relevant feature(s) | Reference/source |
|---|---|---|---|
| pND294 | CIp10 | CaURA3 RPS1 integrative plasmid | 42 |
| pND500 | CIp-His1 | CaHIS1 RPS1 integrative plasmid | 16 |
| pND383 | CIp-Arg4 | CaArg4 RPS1 integrative plasmid | This study |
| pND442 | YPB1-ADHp | CaADH1 promoter/URA3/C. albicans ARS | 35 |
| pND354 | CIpHis1-PADH1 yEmRFP | yEmRFP driven by CaADH1 promoter in CIp-His | 16 |
| pND425 | CIpArg4-PTEF CaCas9 | Codon-optimized Cas9 driven by AgTEF1 promoter in CIp-Arg | This study |
| pND459 | YPB-PADH1 HH gRFP HDV | PADH1HH gRFP HDV in YPB | This study |
| pND465 | YPB-PADH1 HH gLeu2 HDV | PADH1HH gLeu2 HDV in YPB | This study |
| pND468 | YPB-PADH1 tA gRFP HDV | PADH1tRNA gRFP HDV in YPB | This study |
| pND479 | YPB-PtRNA gRFP HDV | PtRNA gRFP HDV in YPB | This study |
| pND474 | pV1090 | PSNR52-gRNA/SATR integrative plasmid | 1 |
| pND476 | pV1090-gRFP | PSNR52-gRFP in pV1090 | This study |
| pND489 | pV1025 | CaCas9/SAT flipper ENO1 integrative plasmid | 1 |
| pND486 | CIp10-PADH1 HH gRFP HDV | PADH1HH sgRFP HDV in CIp10 | This study |
| pND482 | CIp10-PADH1 tA gRFP HDV | PADH1tRNA sgRFP HDV in CIp10 | This study |
| pND483 | CIp10-PtRNA gRFP HDV | PtRNA sgRFP HDV in CIp10 | This study |
| pND484 | CIp10-PSNR52 gRFP | PSNR52 sgRFP in CIp10 | This study |
| pND494 | CIp10-PADH1 tA SapI HDV | CIP10-based cloning vector for ligation of gRNA PADH1tA-SapI2× HDV in CIp10 | This study |
| pND499 | CIp10-PADH1 tA gLEU2 HDV | PADH1tRNA sgLeu2 HDV in CIp10 | This study |
| pND501 | CIp-dpl-PADH1 tA SapI HDV | pND494 but contains ura3-dpl200 instead of URA3 | This study |
DNA sequence of plasmids, gRNA, and expression cassettes used in this study. sgRNA and promoters are color coded according to Fig. 3. Download TABLE S1, PDF file, 0.1 MB (82KB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
CIp-His1-PADH1 yEmRFP (pND354) is a HIS1 integration plasmid that contains the C. albicans codon-optimized yEmRFP (15). CIp-ARG4 (pND383) was constructed by replacing the URA3 gene in CIp10 (35) with a 1-kb BamHI/SacI fragment containing C. albicans ARG4. Unless otherwise noted, CIp plasmids were linearized with StuI to target integration at RPS1.
A C. albicans codon-optimized CAS9 gene, encoding CaCas9 endonuclease with a hemagglutinin (HA) epitope tag and nuclear localization signal at the C terminus and driven by the strong Ashbya gossypii TEF1 promoter, was synthesized (Genescript, NJ) and cloned into CIp-ARG4 as a KpnI/SacI fragment to produce CIp-ARG4-PTEF CaCAS9 (pND425). YPB1-ADHp (pND442) is a 2µ CaURA3 CaARS plasmid that contains the ADH1 promoter and terminator (35).
YPB-PADH1 HH gRFP HDV (pND459) contains an sgRNA that targets the RFP PAM at position +132. sgRNA expression is driven by the RNA polymerase II (Pol II) ADH1 promoter (PADH1); the 5′ and 3′ ends are flanked by the self-cleaving hammerhead (HH) (36) and hepatitis delta virus (HDV) (37) ribozymes, respectively. A 229-bp BglII/MluI fragment (Table S1) containing the HH-gRFP-HDV sequence was synthesized and cloned into the BglII/MluI fragment of YPB1-ADHp, downstream of PADH1.
YPB-PADH1 HH gLeu2 HDV (pND465) is identical to YPB-PADH1 HH gRFP HDV but contains a gRNA that targets the CaLEU2 PAM site at position +123 and an HH sequence whose 6 nucleotides at the 5′ end are complementary to the first 6 nucleotides of gLeu2 (Fig. S1). The HH-gLeu2-HDV cassette was synthesized (Genescript, NJ), and cloned into YPB1-ADHp vector as a BglII/MluI fragment.
YPB-PADH1 tA gRFP HDV (pND468) contains the 75-bp C. albicans tRNAAla gene between the ADH1 promoter and the sgRNA in YPB1-Adhp. The tRNA gene was amplified by PCR from genomic DNA. Gibson assembly (38) was used to assemble the tRNA-sgRNA-HDV fragments and the YPB-ADHp vector.
YPB-PtRNA gRFP HDV (pND479) was generated by deleting the ADH1 promoter of YPB-PADH1 tA gRFP HDV (pND468) by digestion with NotI and BglII, filling in overhangs with Klenow DNA polymerase and ligating.
To construct the CIp10 series of sgRNA delivery plasmids, each of the sgRNA cassettes described above (PADH1 HH gRFP-HDV [1,113 bp], PADH1 tA gRFP-HDV [1,147 bp], and PtRNA gRFP HDV [546 bp]) was cloned into CIp10 as SalI/MluI fragments to produce pND486, pND482, and pND483, respectively. To construct CIp10-PSNR52 gRFP, the gRFP gRNA was first cloned into pV1025 as described previously (1). The 1,112-bp PSNR52 gRFP-tracr fragment was then amplified by PCR using primers with SalI and MluI sites and inserted into CIp10 to produce CIp10-PSNR52 gRFP (pND484).
CIp10-PADH1 tA SapI HDV (pND494) is a vector that allows cloning and expression of any gRNA such that the sgRNA transcript is flanked with 5′ tRNA and 3′ HDV and transcribed by PADH1. It was constructed by using site-directed mutagenesis to replace the RFP gRNA segment in CIp10-PADH1 tA gRFP HDV with a cassette containing two SapI sites and 22 bp of intervening sequence, including a ClaI site (Fig. S2). In addition, site-directed mutagenesis replaced the unique SapI site at position 4381 of CIp10-PADH1 tA gRFP HDV with an NsiI site (Fig. S2). It should be noted that while constructing this plasmid, we discovered that the CIp10 sequence (GenBank accession no. AF181970) between KpnI and SacI (containing pBluescript KS+ sequence) was incorrectly annotated and actually flipped, which places the T7 promoter adjacent to RPS1 and the T3 promoter adjacent to CaURA3. To allow sequential introduction of additional gRNAs into the same strain, we also constructed a plasmid that is identical to pND494 but contains the recyclable ura3-dpl200 allele (26). This URA3 is flanked by 200-bp repeats that facilitate its recycling by FOA selection.
Strains and growth conditions.
C. albicans strains were grown in standard rich YPAD medium (2% Bacto-peptone, 2% dextrose, 1% yeast extract, 20 mg/liter adenine) or synthetic dropout (SD) (2% dextrose, 2% Difco yeast nitrogen base with ammonium sulfate) supplemented with the appropriate nutritional requirements. Uridine (75 mg/liter) was added to all media except SD(−Ura).
The C. albicans strains used in this study are listed in Table 4 and were derived from BWP17. EPC1 contains a single integrated copy of the RFP gene (16) and was constructed by targeting StuI-linearized CIp-HIS-PADH-RFP to RPS1. This integration results in a duplication of RPS1 flanking PADH1-RFP, HIS1 and the intervening plasmid sequence. Single integration of PADH1-RFP at RPS1 was confirmed by Southern blotting (not shown). EPC2, which expresses both RFP and CaCAS9, was constructed by targeting CIp-ARG-PTEF1-CaCas9 (see below) in a second round of integration into the second RPS1 allele in EPC1. An isogenic strain, RJY54 that expresses CaCAS9 but not RFP, was constructed by targeting CIp-ARG-PTEF1-CaCas9 (see below) to RPS1. HNY30 (eno1Δ::Cas9) was constructed by targeting integration of the KpnI/SacI Cas9/SAT-flipper cassette of pV1025 (1) in BWP17 and then plating SATR colonies on YPAD to screen for SATS strains that lost the SAT-flipper cassette. HNY25 was constructed by knocking out LEU2 in RJY54 using the URA3-marked CRISPR/Cas gLEU2 plasmid p465. After confirming the homozygous leu2 mutation by PCR, uracil auxotrophs were selected on plates containing 5-fluoroorotic acid (FOA) and further screened for spontaneous loss of CIp-ARG-PTEF1-CaCas9 by arginine auxotrophy. HNY31 (leu2Δ/leu2Δ eno1Δ::Cas9) was constructed by targeting integration of the KpnI/SacI Cas9/SAT-flipper cassette of pV1025 in HNY25 and screening for SAT sensitivity.
TABLE 4 .
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| BWP17 | ura3Δ::λ imm434/ura3Δ::λ imm434 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG | 27 |
| EPC1 | BWP17 RPS1::PADH1 RFP-HIS1 RPS1 | This study |
| EPC2 | BWP17 RPS1::PADH1 RFP-HIS1-RPS1::PTEF1 CaCas9-HA-ARG4-RPS1 | This study |
| RJY54 | BWP17 RPS1::PTEF1 CaCas9-HA-ARG4-RPS1 | This study |
| HNY25 | BWP17 and leu2Δ/leu2Δ | This study |
| HNY30 | BWP17 eno1Δ::CaCas9 | This study |
| HNY31 | BWP17 eno1Δ::CaCas9 leu2Δ/leu2Δ | This study |
Yeast transformation and quantitation of RFP cleavage.
Yeast transformations were performed by the lithium acetate protocol (39) with the following modifications. Fresh overnight cultures (12 to 16 h) were diluted 1:20 and incubated for ~5 h (optical density at 600 nm [OD600] of 5). Five milliliters was harvested, washed once with H2O and once with 100 mM lithium acetate (LiOAc), and resuspended in 500 µl 100 mM LiOAc. The concentration of plasmid DNA was titrated to yield ~200 colonies per plate. Typically, transformations included 50 µl of the cell suspension (2.5 OD600 units), ~2 to 4 µg of linearized sgRNA plasmid (1 pmol), 1 to 2 µg of RFP donor repair DNA fragment (15 pmol), or annealed, filled-in repair oligonucleotides (200 pmol). After transformation, plates were incubated at 30°C for 2 days before being viewed by fluorescence microscopy with a Zeiss microscope equipped with low-power magnification (1.5 to ×10). Visual detection of pink (RFP) and white (rfp) colonies required 3 days of incubation. The number of red, white, and sectored colonies per plate was counted to determine the efficiency of Cas9-mediated cleavage. Cleavage efficiency was calculated as the no. of white colonies/total no. of colonies per plate.
PCR analysis of Cas9-mediated cleavage.
For analyses of RFP mutagenesis, white colonies were patched and replica plated on selective media to test for prototrophy of selective markers (URA3, HIS1, and ARG4). Genomic DNA from 20 white colonies per experiment was prepared and used as the template for PCR amplification using primers specific for RFP (Fig. S1). Colonies were inoculated into 0.5 ml medium and incubated at 30°C with shaking for 2 to 3 h. After harvesting of cells, pellets were suspended in 30 µl 0.2% SDS, heated for 3 min at 95°C, and centrifuged for 1 min at 14,000 × g. Three microliters of the supernatant was used as genomic DNA template in a standard 25-µl PCR.
For the analyses of LEU2 deletions, after transformation with various sgLEU2 gRNA plasmids or control vectors, and with or without donor repair fragments, colonies were patched onto to SD(−Ura) and replica plated onto SD(−Ura) and SD(−Leu) plates. PCR amplification of Leu2+ and Leu2− colonies was performed, followed by digestion with EcoRI to determine the percentage of colonies that were heterozygous or homozygous for the leu2Δ allele.
Construction of donor healing fragments.
The rfpΔ repair fragment targeted deletion of nucleotides 55 to 402 of the RFP ORF, including the PAM site located at +132. It was generated by fusion PCR (40). PCR was used to amplify two fragments: one homologous to the 5′ region of RFP and the other homologous to the 3′ region. The 5′ fragment also contained a 3′ 20-bp tail that was homologous to the 5′ end of the second fragment (Fig. 2A). These two fragments were mixed, annealed, and then extended. After extension, the full-length “fused” fragment was amplified by PCR. The resulting 593-bp fragment contains a 285-bp arm of upstream sequence homology and a 308-bp arm of downstream sequence homology to regions flanking the DSB. Approximately 1 to 2 µg of this DNA (~5 pmol) was used for transformation of yeast. Recombination with RFP results in deletion of an internal 370 bp within the RFP ORF, including the PAM site to produce the rfpΔ33-403 allele.
The LEU2 donor repair fragment was made by annealing and filling in two 60-mer nucleotides that contained 20 bases of sequence complementarity at their 3′ ends. This complementary region included an EcoRI recognition sequence (highlighted in blue in Fig. 7A). The resulting fragment contained 47 bp of homology to sequences flanking the DSB. Three microliters of each oligonucleotide (300 pmol) was annealed and then extended in a 25-µl reaction mixture containing 0.2 mM deoxynucleoside triphosphate (dNTP), buffer, and Taq DNA polymerase (Denville Scientific, Inc., United States) and subjected to 25 to 30 cycles of PCR with an extension time of 30 s. Twenty microliters of this reaction mixture was used per yeast transformation as a repair donor fragment (~240 pmol of repair fragment). Recombination with LEU2 results in replacement of an internal 434-bp fragment within the LEU2 ORF, including the PAM site, with an EcoRI site to produce the leu2Δ71-505 allele.
ACKNOWLEDGMENTS
We thank Eric Paullisen, Rachel Jones, and Katiuska Daniela Pulgar Prieto for their technical contributions to this study and Valmik Vyas for plasmids.
H.N. was supported in part by a URECA-Biology Alumni Research award. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Vyas VK, Barrasa MI, Fink GR. 2015. A Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Sci Adv 1:e1500248. doi: 10.1126/sciadv.1500248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 3.Garneau JE, Dupuis MÈ, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S. 2010. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 4.Gasiunas G, Barrangou R, Horvath P, Siksnys V. 2012. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A 109:E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. 2013. RNA-guided human genome engineering via Cas9. Science 339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donoho G, Jasin M, Berg P. 1998. Analysis of gene targeting and intrachromosomal homologous recombination stimulated by genomic double-strand breaks in mouse embryonic stem cells. Mol Cell Biol 18:4070–4078. doi: 10.1128/MCB.18.7.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storici F, Durham CL, Gordenin DA, Resnick MA. 2003. Chromosomal site-specific double-strand breaks are efficiently targeted for repair by oligonucleotides in yeast. Proc Natl Acad Sci U S A 100:14994–14999. doi: 10.1073/pnas.2036296100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. 2013. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan OW, Skerker JM, Maurer MJ, Li X, Tsai JC, Poddar S, Lee ME, DeLoache W, Dueber JE, Arkin AP, Cate JH. 2014. Selection of chromosomal DNA libraries using a multiplex CRISPR system. Elife 3:e03703. doi: 10.7554/eLife.03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min K, Ichikawa Y, Woolford CA, Mitchell AP. 2016. Candida albicans gene deletion with a transient CRISPR-Cas9 system. mSphere 1:e00130-16. doi: 10.1128/mSphere.00130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marck C, Kachouri-Lafond R, Lafontaine I, Westhof E, Dujon B, Grosjean H. 2006. The RNA polymerase III-dependent family of genes in hemiascomycetes: comparative RNomics, decoding strategies, transcription and evolutionary implications. Nucleic Acids Res 34:1816–1835. doi: 10.1093/nar/gkl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res 41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keppler-Ross S, Noffz C, Dean N. 2008. A new purple fluorescent color marker for genetic studies in Saccharomyces cerevisiae and Candida albicans. Genetics 179:705–710. doi: 10.1534/genetics.108.087080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keppler-Ross S, Douglas L, Konopka JB, Dean N. 2010. Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot Cell 9:1776–1787. doi: 10.1128/EC.00156-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Zhao Y. 2014. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol 56:343–349. doi: 10.1111/jipb.12152. [DOI] [PubMed] [Google Scholar]
- 18.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. 1983. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 19.Schiffer S, Rösch S, Marchfelder A. 2002. Assigning a function to a conserved group of proteins: the tRNA 3′-processing enzymes. EMBO J 21:2769–2777. doi: 10.1093/emboj/21.11.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie K, Minkenberg B, Yang Y. 2015. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci U S A 112:3570–3575. doi: 10.1073/pnas.1420294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugawara N, Ira G, Haber JE. 2000. DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol Cell Biol 20:5300–5309. doi: 10.1128/MCB.20.14.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore JK, Haber JE. 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol 16:2164–2173. doi: 10.1128/MCB.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth DB, Wilson JH. 1986. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol 6:4295–4304. doi: 10.1128/MCB.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legrand M, Chan CL, Jauert PA, Kirkpatrick DT. 2007. Role of DNA mismatch repair and double-strand break repair in genome stability and antifungal drug resistance in Candida albicans. Eukaryot Cell 6:2194–2205. doi: 10.1128/EC.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciudad T, Andaluz E, Steinberg-Neifach O, Lue NF, Gow NA, Calderone RA, Larriba G. 2004. Homologous recombination in Candida albicans: role of CaRad52p in DNA repair, integration of linear DNA fragments and telomere length. Mol Microbiol 53:1177–1194. doi: 10.1111/j.1365-2958.2004.04197.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RB, Davis D, Enloe BM, Mitchell AP. 2000. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast 16:65–70. doi:. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RB, Davis D, Mitchell AP. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181:1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellington M, Rustchenko E. 2005. 5-Fluoro-orotic acid induces chromosome alterations in Candida albicans. Yeast 22:57–70. doi: 10.1002/yea.1191. [DOI] [PubMed] [Google Scholar]
- 29.Doench JG, Hartenian E, Graham DB, Tothova Z, Hegde M, Smith I, Sullender M, Ebert BL, Xavier RJ, Root DE. 2014. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol 32:1262–1267. doi: 10.1038/nbt.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Xiao T, Chen CH, Li W, Meyer CA, Wu Q, Wu D, Cong L, Zhang F, Liu JS, Brown M, Liu XS. 2015. Sequence determinants of improved CRISPR sgRNA design. Genome Res 25:1147–1157. doi: 10.1101/gr.191452.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS. 2014. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farboud B, Meyer BJ. 2015. Dramatic enhancement of genome editing by CRISPR/Cas9 through improved guide RNA design. Genetics 199:959–971. doi: 10.1534/genetics.115.175166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radzisheuskaya A, Shlyueva D, Müller I, Helin K. 2016. Optimizing sgRNA position markedly improves the efficiency of CRISPR/dCas9-mediated transcriptional repression. Nucleic Acids Res 44:e141. doi: 10.1093/nar/gkw583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JD, Suresh S, Schlecht U, Wu M, Wagih O, Peltz G, Davis RW, Steinmetz LM, Parts L, St Onge RP. 2016. Quantitative CRISPR interference screens in yeast identify chemical-genetic interactions and new rules for guide RNA design. Genome Biol 17:45. doi: 10.1186/s13059-016-0900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertram G, Swoboda RK, Gooday GW, Gow NA, Brown AJ. 1996. Structure and regulation of the Candida albicans ADH1 gene encoding an immunogenic alcohol dehydrogenase. Yeast 12:115–127. [DOI] [PubMed] [Google Scholar]
- 36.Scott WG, Finch JT, Klug A. 1995. The crystal structure of an all-RNA hammerhead ribozyme: a proposed mechanism for RNA catalytic cleavage. Cell 81:991–1002. doi: 10.1016/S0092-8674(05)80004-2. [DOI] [PubMed] [Google Scholar]
- 37.Nakano S, Chadalavada DM, Bevilacqua PC. 2000. General acid-base catalysis in the mechanism of a hepatitis delta virus ribozyme. Science 287:1493–1497. doi: 10.1126/science.287.5457.1493. [DOI] [PubMed] [Google Scholar]
- 38.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 39.Walther A, Wendland J. 2003. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr Genet 42:339–343. doi: 10.1007/s00294-002-0349-0. [DOI] [PubMed] [Google Scholar]
- 40.Higuchi R, Krummel B, Saiki RK. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res 16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darty K, Denise A, Ponty Y. 2009. Varna: interactive drawing and editing of the RNA secondary structure. Bioinformatics 25:1974–1975. doi: 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murad AM, Lee PR, Broadbent ID, Barelle CJ, Brown AJ. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
pADH/tRNA plasmids used for high-efficiency gRNA expression. (A) Schematic diagram of the CIp-PADH/tRNA vector that contains a cloning cassette containing two nonpalindromic SapI sites, separated by a unique ClaI, at the tRNA-tracrRNA junction. These RPS1 integration plasmids are marked with URA3 (p494) or ura3-dpl200 (p501). (B) The sequence of the SapI cassette (blue) at the tRNA (green)-tracrRNA (purple) junction. The positions of ClaI and SapI sites within the cassette are underlined, and arrows depict the SapI cleavage sites. Beneath the SapI cassette is an example of how to design two gRNA oligonucleotides (boxed, using LEU2 gRNA). Note the gRNA (red) oligonucleotides contain CAA overhangs that create sticky ends when annealed, which allow ligation of these oligonucleotides into SapI-digested vector. Ligation results in recreation of the RNase Z tRNA recognition cleavage site, as well as the correct fusion of the gRNA with the tracrRNA. Correct ligation products are screened by the loss of the ClaI site. Download FIG S1, PDF file, 1.8 MB (1.9MB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
NHEJ DNA sequence trace. Shown is a partial DNA trace of an rfp mutant allele whose sequence is consistent with an NHEJ event. A schematic diagram of a portion of the sequence aligned to RFP is depicted, showing deletion endpoints. (See the sequence alignment in Fig. 6B.) Download FIG S2, PDF file, 0.2 MB (184.4KB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Timeline for CRISPR/Cas mutagenesis in C. albicans. Download FIG S3, PDF file, 0.1 MB (80.4KB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DNA sequence of plasmids, gRNA, and expression cassettes used in this study. sgRNA and promoters are color coded according to Fig. 3. Download TABLE S1, PDF file, 0.1 MB (82KB, pdf) .
Copyright © 2017 Ng and Dean.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.