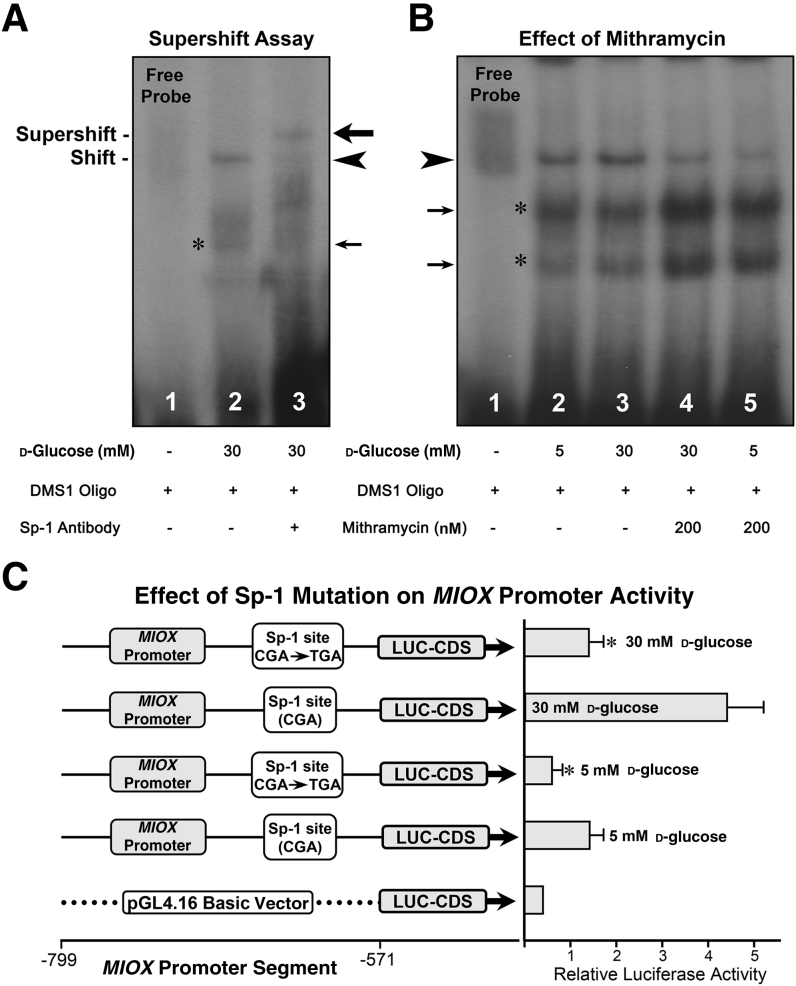
Figure 3.
Specificity of specificity protein (Sp)-1–binding with human MIOX gene and modulation of its promoter activity. A: The specificity of binding of differentially methylated segment (DMS)-1 oligo was assessed by using anti–Sp-1 antibody in eletrophoretic mobility shift assays. A distinct band is seen when nuclear extracts are incubated with DMS1 (arrowhead). On subsequent incubation with the antibody, the band shifts to higher position (supershift), suggesting the formation of a DMS1-DNA:nuclear protein:Sp-1 antibody complex, and binding seems to be specific (big arrow). B: Validation of Sp-1 binding was assessed with the use of the Sp1 gene–specific inhibitor mithramycin. An increase in the intensity of the band is observed with the incubation of DMS1 oligo under high-glucose (30 mmol/L) ambience (arrowhead). Co-treatment of HK-2 cells with 200 nm of mithramycin notably reduces the intensity of the band under high-glucose ambience, whereas its intensity is drastically reduced under low glucose (5 mmol/L) ambience. C: Whether Sp-1 modulates the MIOX promoter activity was assessed by mutational analyses, where mutant reporter constructs were generated. Under low-glucose ambience, a mild relative luciferase (LUC) activity is observed, and it is notably reduced with a single base pair mutation (C → T) within the MIOX promoter. Under high-glucose ambience, a remarkable increase in the promoter activity is observed, and it is markedly reduced with the mutation in the Sp-1–binding site. The asterisks and small arrows in A and B indicate nonspecific bands. Data are expressed as means ± SEM. n = 4 independent experiments (C). ∗P < 0.05 versus the respective control. CDS, coding segment.