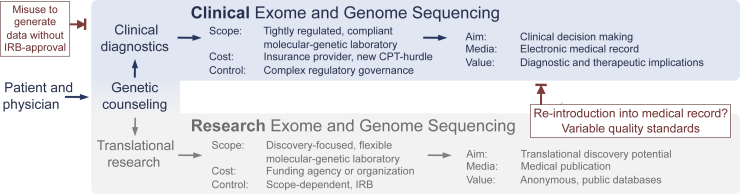
Figure 1.
Comparison between clinical and research exome and genome sequencing. The scope, cost, and regulatory governance differ substantially between translational research (bottom) and clinical diagnostics (top). In contrast to the broad adoption of exome and genome sequencing by the scientific community, the administrative burden, regulatory complexity, and unresolved reimbursement situation in clinical diagnostics can appear as an unnecessary hurdle to an otherwise easily available test. The lack of a functioning clinical workflow has turned the institutionally review board–approved, patient-consented approach (bottom) into a pathway to re-introduce research-based findings into the medical record (bottom right, blocked red arrow). In reverse, utilization management is needed to oversee the clinical diagnostic route to prevent misuse (top left, blocked red arrow) of clinical data for research studies without appropriate institutional review. The comparison shows the need for a minimum viable workflow for clinical-grade, reimbursable exome and genome sequencing. CPT, Current Procedural Terminology (medical code set maintained by the American Medical Association through the CPT Editorial Panel); IRB, institutional review board/ethics committee.