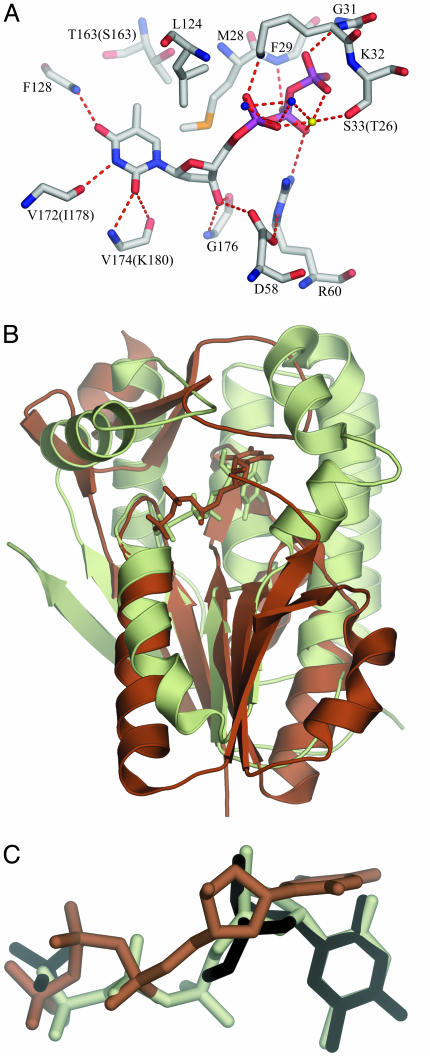
Fig. 3.
Binding interactions of dTTP at the active site as found in hTK1. (A) The active-site amino acids are well conserved; only the residues that differ in Uu-TK are in brackets. The hydrogen-bond donors and acceptors of the base all form hydrogen bonds to main-chain atoms: O2 and N3 bond to main-chain atoms of the lasso loop, and O4 bonds to main-chain nitrogen of the α/β-domain. The methyl group of thymine is positioned in a hydrophobic pocket directed toward the β-carbon of a Ser in Uu-TK and a Thr in hTK1 surrounded by Met, Leu, and Tyr. The 3′-oxygen atom of the deoxyribose is hydrogen-bonded to the amino group of a conserved Gly in the lasso domain and to the side chain of the conserved Asp in the flexible loop of the α/β-domain. (B) Comparison of hTK1 and Dm-dNK with dTTP. hTK1 (brown) was superimposed with Dm-dNK bound with dTTP (light green). The P loop and the flanking regions were used in the superpositioning. (C) The thymidine base in hTK1 is bound perpendicularly compared with the way it is bound in Dm-dNK. dT and the sulfate ion bound to Dm-dNK are shown in black, dTTP bound to hTK1 is shown in brown, and dTTP bound to Dm-dNK is shown in light green.