Abstract
Background
Depression is a highly prevalent co-morbidity in Chronic Obstructive Pulmonary Disease (COPD) which was shown to be associated with a worse course of disease, including reduced quality of life and increased symptoms burden, healthcare use, and even mortality. It has been speculated that systemic inflammation may play a role in the presence of depression. Currently, physical activity is an important lifestyle factor that has the potential to modify inflammatory cytokines and depression, however our understanding of how to use exercise effectively in COPD patients to alleviate depression related systemic inflammation is incomplete and has prompted our interest to identify the type and intensities of effective exercise.
Objective
The aim of this study was to measure the changes in depression related systemic inflammation of aerobic exercise training in COPD patients in Jeddah area.
Material and methods
Eighty patients with moderate severity of COPD participated in this study and were divided into two groups; the first group received aerobic exercise, whereas the second group received no exercise training for 12 weeks.
Results
The mean values of tumor necrosis factor-alpha (TNF-α), interleukin-4 (IL-4), interleukin-6 (IL-6), C-reactive protein (CRP) and Beck Depression Inventory (BDI) scores were significantly decreased in in group (A) after treatments, but the changes in group (B) were not significant .Also, there were significant differences between mean levels of the investigated parameters in group (A) and group (B) at the end of the study.
Conclusion
Aerobic exercise is an effective treatment policy to improve depression related to systemic inflammation in patients with chronic obstructive pulmonary disease.
Keywords: Aerobic exercise, depression, inflammatory cytokine, chronic obstructive pulmonary disease
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a highly prevalent chronic lung disease Worldwide. The prevalence is variable between countries, but overall there is a prevalence rate of around 10% in individuals aged 40 and above1. In developed countries, COPD is responsible for approximately 4% of all deaths and is the only major condition for which the burden of disease continues to increase, currently being 5th overall in underlying cause of death and 3rd for burden of disease2,3.
Chronic Obstructive Pulmonary Disease (COPD) is a medical condition with multiple co-morbidities4,5. One of the most common is depression that occurs in 10 to 42% of persons with COPD and is associated with low quality of life6. Depression is associated with increased frequency of hospital admissions, prolonged length of stay, increased number of consultations, low compliance with medical treatment and premature death7,8. Patients with COPD have a higher prevalence of depression and anxiety than the general population9,10 and COPD patients have relative risk of 1.69 of developing depression11.
Patients with depression may not adhere to their management programs such as the pulmonary rehabilitation and smoking cessation. Therefore, depression may lead to poor clinical outcomes. It has been shown that depression leads to higher health care use with higher admission and relapse rate in emergency department patients. Ultimately, the presence of depression in patients with COPD leads to higher economic burden12,13.
Chronic obstructive pulmonary disease and depression are significantly associated due to multiple reasons. About 40% of patients with COPD are found to have depression, compared to a prevalence of about 15–20 % in the general population14. We found a prevalence of close to 90% of at least mild depression (as measured on the Hamilton depression scale) in patients admitted with COPD15. Loss of independence with increasing disability in COPD can cause, or aggravate, depression. A predisposition to depression may increase the risk of smoking, as nicotine has a mood elevating effect. Systemic inflammation may also play a role in depression16. Systemic inflammation biomarkers include interleukin-6 (IL-6) and C-reactive protein (CRP) have been shown to be elevated in individuals with depression17–19 and decreased after antidepressant treatment20.
Exercise is a readily available therapeutic option, effective as a first-line treatment in mild to moderate depression21. Additionally, exercise has a utility in preventing depression and has beneficial effects on other common co-morbidities (i.e. cardiovascular disease risk factors and glycemic control). A prospective, randomized controlled trial found that exercise was as effective as Sertraline (selective serotonin reuptake inhibitor) for the treatment of depression - the effect size of exercise was 2.022. Several reviews show exercise compares favorably to antidepressants and cognitive behavioral therapy as a first-line treatment for mild to moderate depression23.
Aerobic exercise training is hypothesized to improve depression related to systemic inflammation but evidence is scarce, so this study aimed to measure depression and systemic inflammation response to aerobic exercises in patients with chronic obstructive pulmonary disease.
Patients and methods
Subjects
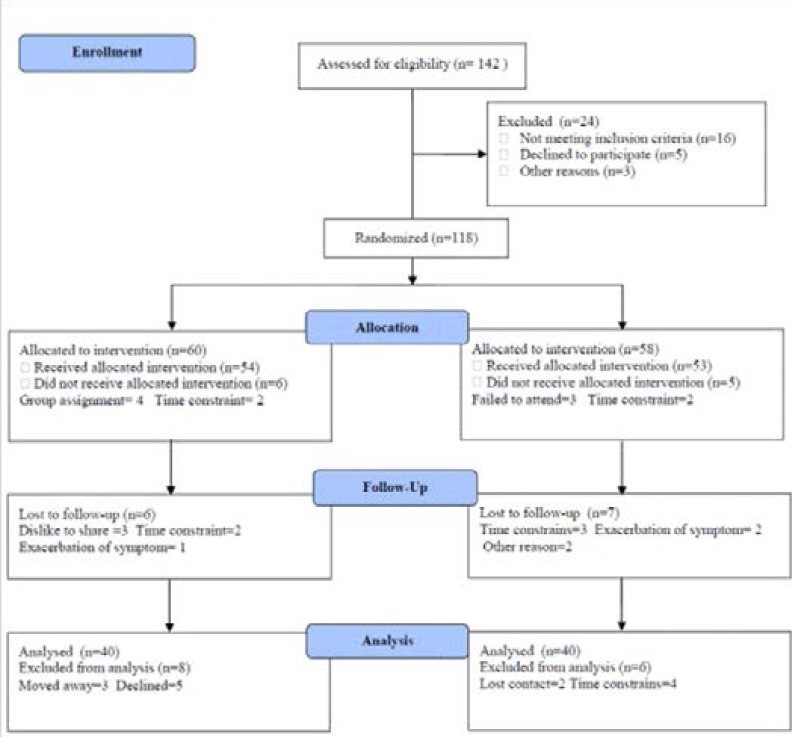
Eighty patients with moderate severity of chronic obstructive pulmonary disease according to GOLD24 were enrolled in this study. Patients with exacerbations in the last 4 weeks were either rescheduled or excluded; their age ranged from 35 to 55 years. Exclusion criteria included ischemic heart disease, congestive heart disease, cerebrovascular disease, dementia, lung cancer, known psychiatric illness, maintenance treatment with systemic corticosteroids (oral, parenteral), active tuberculosis, inflammatory bowel syndrome or insulin dependent diabetes mellitus. Participants were divided into two groups, the first group received aerobic exercises, while the second group was considered as a control group and received no training intervention for three months. The CONSORT diagram outlining the details of the screening, run-in and randomization phases of the study and reasons for participant exclusion can be found in figure (1). Informed consent was obtained from all participants. This study was approved by the Scientific Research Ethical Committee, Faculty of Applied Medical Sciences at King University.
Measurements
1. Inflammatory cytokines measurements: Blood sample was drained from the antecubital vein after a 12-h fasting, the blood samples were centrifuged at + 4 °C (1000 = g for 10 min). Interleukin-6 (IL-6), C-reactive protein (CRP) levels were analyzed by “Immulite 2000”. However, tumor necrosis factor-alpha (TNF-α) level was measured by ELISA kits (ELX 50). All analyses were done by Hitachi 7170 Autoanalyser (Tokyo, Japan) and kits (Randox).
2. Beck depression inventory (BDI): It measures the depth and behavioral manifestations of depression and consists of 21 items, each of which has four responses of increasing severity. Numerical values from 0–3 were assigned to each statement to indicate the degree of severity. A total score from 0–9 was considered normal, 10–16 reflected mild depression, 17–29 reflected moderate depression and 30 or above was considered severe depression25.
Procedures
Following the previous evaluation, all patients were divided randomly into the following groups:
1. Patients in Group (A) were submitted to a 40 min aerobic session on a treadmill (the initial, 5-minute warm-up phase performed on the treadmill (Track master 400E, gas fitness system, England) at a low load, each training session lasted for 30 minutes and ended with 5-minute recovery and relaxation phase) either walking or running, based on heart rate, until the target heart rate was reached, according to American College of Sport Medicine guidelines. The program was started with 10 min of stretching and was conducted using the maximal heart rate index (HRmax) estimated by: 220-age. First 2 weeks = 60–70% of HRmax, 3rd to 12th weeks = 70–80% of HRmax.26. However, some participants experienced adverse events included attacks of breathlessness (dyspnea), muscle cramp and soreness due to lack of exercise tolerance specially at the beginning of the training program.
2. Patients in Group (B) received no exercise training.
Statistical analysis
Statistical analysis of data was performed using SPSS (Chicago, IL, USA) version 17. The mean values of the investigated parameters obtained before and after three months in both groups were compared using paired “t" test. Independent “t” test was used for the comparison between the two groups. The degree of correlation between BDI scores and cytokine levels was detected by Pearson's product moment correlation coefficients (r). All data were expressed as the mean ± SD. P<0.05 indicated statistical significance.
Results
The baseline characteristics of all participants are shown in Table (1). Most participants (65%) were men. Forty participants were assigned to the aerobic exercise group (n = 40; 26 males and 14 females), while the resistance exercise group had (n = 40; 27 males and 13 females). None of the baseline characteristics differed significantly between the two groups is listed in table (1).
Table (1).
Mean value of demographic data for participants in both groups.
| Mean +SD | Significance | ||
| Group (A) | Group (B) | ||
| Age (year) | 33.73 ± 5.14 | 34.61 ± 4.82 | P>0.05 |
| Gender ratio (male/female) | 26/14 | 27/13 | P>0.05 |
| Weight (kg) | 63.15 ± 6.17 | 60.98 ± 5.72 | P>0.05 |
| Height (cm) | 162.32 ± 8.64 | 160.51 ± 7.68 | P>0.05 |
| BMI (kg/m2) | 22.83 ± 3.57 | 20.88 ± 3.16 | P>0.05 |
| FVC (L) | 2.51 ± 0.96 | 2.43 ± 0.85 | P>0.05 |
| FEV1 (L) | 1.47 ± 0.63 | 1.29 ± 0.56 | P>0.05 |
| FEV1/FVC (%) | 48.86 ± 9.12 | 47.53 ± 8.44 | P>0.05 |
| MVV (L/minute) | 47.16 ± 10.87 | 45.11 ± 9.58 | P>0.05 |
| Total CAT score | 19.36 ± 4.15 | 19.17 ± 4.23 | P>0.05 |
| COPDSS | 5.97 ± 2.54 | 6.18 ± 2.46 | P>0.05 |
BMI: Body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; FEV1/FVC: Ratio between forced expiratory volume in the first second and forced vital capacity; MVV: Maximum voluntary ventilation; CAT: The COPD Assessment Test; COPDSS: chronic obstructive pulmonary disease severity score.
The mean values of TNF-α, IL-4, IL-6, CRP and Beck Depression Inventory (BDI) scores were significantly decreased in group (A) at the end of the study (table 2), but the changes in group(B)were not significant (table 3).
Table (2).
Mean value and significance of TNF-α, IL-4, IL-6,CRP and BDI scores in group (A) before and at the end of the study.
| Mean +SD | T-value | Significance | ||
| Before | After | |||
| TNF-β (pg/ml) | 6.46 ± 1.72 | 4.53 ± 1.28* | 7.12 | P<0.05 |
| IL-4(pg/ml) | 5.71 ± 1.63 | 3.45 ± 1.52* | 6.55 | P<0.05 |
| IL-6 (pg/ml) | 8.19 ± 2.51 | 5.27 ± 1.88* | 6.84 | P<0.05 |
| CRP(mg/dl) | 15.34 ± 3.26 | 9.85 ± 2.44* | 7.31 | P<0.05 |
| BDI scores | 8.26 ± 2.11 | 5.24 ± 1.97* | 6.15 | P < 0.05 |
TNF- α: tumor necrosis factor - alpha; IL-4: Interleukin-4; IL-6: Interleukin-6; CRP: C-reactive protein ; BDI: Beck Depression Inventory;
indicates a significant difference between the two groups, P < 0.05.
Table (3).
Mean value and significance of TNF-α, IL-4, IL-6, CRP and BDI scores in group (B) before and at the end of the study.
| Mean +SD | T-value | Significance | ||
| Before | After | |||
| TNF-α (pg/ml) | 6.38 ± 1.64 | 6.51 ± 1.68 | 0.73 | P>0.05 |
| IL-4(pg/ml) | 5.62 ± 1.47 | 5.74 ± 1.51 | 0.65 | P>0.05 |
| IL-6 (pg/ml) | 8.23 ± 2.65 | 8.41 ± 2.62 | 0.83 | P>0.05 |
| CRP(mg/dl) | 15.75 ± 3.41 | 16.08 ± 3.50 | 0.96 | P>0.05 |
| BDI scores | 8.43 ± 2.32 | 8.65 ± 2.49 | 0.87 | P>0.05 |
TNF- α: tumor necrosis factor - alpha; IL-4: Interleukin-4; IL-6: Interleukin-6; CRP: C-reactive protein; BDI: Beck Depression Inventory.
Also, there were significant differences between mean levels of the investigated parameters in group (A) and group (B) at the end of the study(table 4).
Table (4).
Mean value and significance of TNF-α, IL-4, IL-6, CRP and BDI scores in group (A) and group (B) at the end of the study.
| Mean +SD | T-value | Significance | ||
| Group (A) | Group (B) | |||
| TNF-α (pg/ml) | 4.53 ± 1.28* | 6.51 ± 1.68 | 6.12 | P<0.05 |
| IL-4(pg/ml) | 3.45 ± 1.52* | 5.74 ± 1.51 | 5.36 | P<0.05 |
| IL-6 (pg/ml) | 5.27 ± 1.88* | 8.41 ± 2.62 | 5.27 | P<0.05 |
| CRP(mg/dl) | 9.85 ± 2.44* | 16.08 ± 3.50 | 6.13 | P<0.05 |
| BDI scores | 5.24 ± 1.97* | 8.65 ± 2.49 | 5.22 | P<0.05 |
TNF- α: tumor necrosis factor - alpha; IL-4: Interleukin-4; IL-6: Interleukin-6; CRP:C-reactive protein ; BDI: Beck Depression Inventory;
indicates a significant difference between the two groups, P < 0.05.
However, table 5 summarizes the relationship between BDI scores and cytokine levels in group (A) at the end of the study. Serum levels TNF-α, IL-4, IL-6 and CRP showed a direct relationship with BDI scores (Table 5). These results confirm that aerobic exercise is appropriate to modulate depression related to systemic inflammation in patients with chronic obstructive pulmonary disease.
Table (5).
Correlation coefficient (r) of BDI scores and cytokine levels in group (A) at the end of the study.
Spearman's correlation was used
P < 0.05
P < 0.01
Discussion
Globally, chronic obstructive pulmonary disease (COPD) becomes more prevalent and becomes the third cause of death27,28. However, by 2030 it is expected to have about 9 million patients to die with COPD every year29. Moreover, the economic and health related burden of COPD are enormous30. Inflammation is one of key processes in the pathogenesis of COPD31,32. The inflammatory cytokines as C-reactive protein (CRP), TNF-α, interleukin-1beta (IL-1β) and IL-6 serve as excellent biomarkers when investigating the potential relationship between inflammation and mood disorders33,34. An apparent dose response has been observed with worsening of depressive symptoms correlated with higher levels of inflammatory markers35. To date, there are relatively few adequately-powered, trials of an exercise intervention on depression related inflammatory biomarkers in individuals with COPD. In our study, the mean values of TNF-α, IL-4, IL-6, CRP and BDI were significantly decreased after aerobic exercise training. These results are in line with many previous studies.
Dekker et al. stated that a 12-week exercise intervention resulted in a significant decrease in circulating IL-6 in subjects with type 2 diabetes mellitus who underwent an exercise program without weight loss36. Also, Mikkelsen et al. proved that life-long endurance exercise was associated with a lower level of the inflammatory markers CRP and IL-6 in elderly subjects37. While, Sugawara et al. concluded that the levels of elevated inflammatory cytokines decreased significantly after intervention with an anti-inflammatory nutrition combined with the low-intensity exercise in stable elderly COPD patients38. In addition, there is evidence of lowered IL-6 and TNF-α after prolonged exercise in obese women39 and decreased TNF-α after 12 weeks of aerobic exercise in patients with heart disease40. Moreover, in obese postmenopausal women with type 2 diabetes, 14 weeks of aerobic exercise decreased CRP by 15% and marginally decreased IL-6 (p=0.07)41. Likewise, 12 week of exercise reduced IL-18 levels by 17.5% in patients with metabolic syndrome42. In one of the largest, yet non-randomized, exercise studies conducted to date (HERITAGE Family Study), plasma CRP was significantly reduced with 20 week of aerobic training only in the sub-group of persons with a high baseline CRP43.
The exact mechanisms by which physical activity may reduce inflammation are not entirely understood, there are some data pointing to factors that may contribute to an effect of repeated bouts of muscle contraction leading to improvements in inflammatory status over time44. Exercise training-induced improvements in inflammatory status may also result from the modulation of intracellular signaling path ways and cellular function that are mediated by nitric oxide45. Also, exercise training decrease in mononuclear cell production of atherogenic cytokines (TNF-α and IL-1α), while the production of atheroprotective cytokines (IL-10, IL-4, and transforming growth factor beta-1 (TGFβ1)) increased46. Exercise training also reduces CD14+CD16+ monocyte number, as well as TNFα production by monocytes47 and reduces monocyte cell-surface expression of toll-like receptor-4 (TLR4), a lipopolysaccharide (LPS) signaling receptor that likely contributes to attenuation of acute immune responses to infection or trauma48–50. Similarly, higher-intensity aerobic exercise training reduces stimulated production of TNF-α by monocytes. Thus, these data point to an adaptive down-regulation of cytokine release from innate immune cells in response to regularly performed muscular contraction51,52. Moreover, the potential mechanisms for the anti-inflammatory effect of exercise may include reduced percentage of body fat and macrophage accumulation in adipose tissue, muscle-released interleukin-6 inhibition of tumor necrosis factor-α, and the cholinergic anti-inflammatory pathway53.
Mota-Pereira and colleagues proved that a home-based exercise program of 30–45 min/day walks, 5 days/week for three moths improved depression and functioning parameters in treatment-resistant 150 patients with major depressive disorder, and contributed to remission of 26% of these patients. Moderate intensity exercise may be a helpful and effective adjuvant therapy for treatmentresistant MDD54. Blumenthal et al., had 101 healthy older adults randomized to four months of aerobic exercise, a yoga/flexibility control group, or wait list, assessment of scores from pre- to post-treatment revealed that depressive symptoms were reduced, especially in men55. McNeil et al. designed a study to assess the effects of exercise on total level as well as subtypes of depressive symptoms (i.e., somatic, psychological), a community sample of 30 older adults with “moderate” depression was randomized to one of three conditions: supervised exercise, social contact control, or wait list. Participants in the exercise and social contact groups experienced a significant reduction in total and psychological depressive symptoms relative to wait list participants. Only participants in the exercise condition demonstrated significant improvement in somatic symptomatology following treatment56.
Also, Blumenthal et al. assessed self-reported exercise in a sample of over 2000 men and women (mean age of approximately 60 years) who had suffered a recent myocardial infarction and were either depressed or reported a low level of social support. Patients who reported participation in regular exercise (47% of the sample) had lower depressive symptoms at baseline relative to their sedentary counterparts. In addition, exercisers had lower depression scores six months after they experienced myocardial infarction. Interestingly, exercise participation also was associated with a 50% reduction in mortality over a three-year follow-up period57. Meta-analyses from 2010 by Conn included 70 studies with 2679 clinically depressed subjects and suggested that there was a moderate and statistically significant effect size for exercise in treating depression (supervised exercise effect size is 0.372 and un-supervised exercise effect size is 0.522)58. Another review conducted for the Cochrane review database, with 27 articles in total and 907 participants, showed evidence suggesting exercise was effective in the treatment of depression (standardized mean difference was _0.82, equaling a large clinical effect)23.
There are many possible explanations for antidepressant effects of physical exercise and generally they could be divided in two major groups: “psychological” and “biological” mechanisms. Psychological mechanisms involve improvements in self-esteem, self-efficacy59, self-concept60, improved coping efficacy61 and sleep quality62. By other hand, some of the main biological mechanisms are reduced production of neuro-inflammatory factors, i.e. TNF-α, IL-6, CRP, IL-1b that affect the main neuroimmune mechanisms potentially leading to symptoms of depression-like behavior63–67, also release of Beta endorphins68, the modification in serotonin function proposed by excessive neurotrophins, especially Brain Derived Neurotrophin Factor(BDNF)69–71.
Strengths and limitations
The major strength is the supervised nature of the study. Supervising physical activity removes the need to question compliance or to rely on activity questionnaires. Further, all exercise sessions were supervised and adherence to the activities was essentially 100%. Moreover, the study was randomized; hence, we can extrapolate adherence to the general population. On the other hand, the major limitations is only patients with moderate severity of COPD were enrolled in the study, so the value of this study only related to moderate severity of COPD, also small sample size in both groups may limit the possibility of generalization of the findings in the present study in addition a number of confounders as socioeconomic indicators like previous occupation and income which should be controlled as they can affect the outcomes were not measured. Finally, within the limit of this study, aerobic exercise training is recommended for modulation of low grade systemic inflammation and depression among patients with COPD. Further researches are needed to explore the impact of different therapeutic interventions on quality of life and other biochemical parameters among COPD patients.
Conclusion
The current study provides evidence that aerobic exercise is an effective treatment policy to improve depression related to systemic inflammation in patients with chronic obstructive pulmonary disease.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (36-142-1437-G). The authors, therefore, acknowledge with thanks DSR technical and financial support.
References
- 1.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E. International variation in the prevalence of COPD (the BOLD Study): a populationbased prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare, author. COPD (chronic obstructive pulmonary disease) cited 2013 Dec 28. Available online: http://www.aihw.gov.au/copd/
- 3.Global Strategy for the diagnosis, management and prevention of chronic obstruvtive pulmonary disease Global Initiative for Chronic Obstructive Lung Disease. [December 2013]. Revised 2013. Available online: http://www.goldcopd.org/
- 4.De S. Prevalence of Depression in Stable Chronic Obstructive Pulmonary Disease. Indian J Chest Dis Allied Sci. 2011;53:35–39. [PubMed] [Google Scholar]
- 5.Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011;17(Suppl 1):S21–S28. doi: 10.1097/01.mcp.0000410744.75216.d0. [DOI] [PubMed] [Google Scholar]
- 6.Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, Hanania NA, ACCP Workshop Panel on Anxiety and Depression in COPD Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S–56S. doi: 10.1378/chest.08-0342. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunik ME, Roundy K, Veazey C, Souchek J, Richardson P, Wray NP, Stanley MA. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205–1211. doi: 10.1378/chest.127.4.1205. PubMed. [DOI] [PubMed] [Google Scholar]
- 8.Kim HF, Kunik ME, Molinari VA, Hillman SL, Lalani S, Orengo CA, Petersen NJ, Nahas Z, Goodnight-White S. Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics. 2000;41(6):465–471. doi: 10.1176/appi.psy.41.6.465. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Cafarella PA, Effing TW, Usmani ZA, Frith PA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17:627–638. doi: 10.1111/j.1440-1843.2012.02148.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Pumar MI1, Gray CR1, Walsh JR1, Yang IA1, Rolls TA1, Ward DL1. Anxiety and depression-Important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615–1631. doi: 10.3978/j.issn.2072-1439.2014.09.28. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144:766–777. doi: 10.1378/chest.12-1911. PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122:1633–1637. doi: 10.1378/chest.122.5.1633. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Murali Mohan BV1, Sen T, Ranganath R. Systemic manifestations of COPD. J Assoc Physicians India. 2012;60(Suppl):44–47. [PubMed] [Google Scholar]
- 14.Norwood R, Balkissoon R. Current perspectives on management of co-morbid depression in COPD. Journal of Chronic Obstructive Pulmonary Disease. 2005;2:185–193. doi: 10.1081/copd-200050740. [DOI] [PubMed] [Google Scholar]
- 15.Hibare K, Kamalaksha S, Kalyani N, Asha P, Kirthana K, Murthy P, Murali Mohan BV. Depression in COPD — a depressingly frequent finding!; Paper presented at NAPCON 2003; Coimbatore. [Google Scholar]
- 16.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatoryresponses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a metaanalysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53. doi: 10.1186/1465-9921-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiles SA, Baker AL, de Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a metaanalysis. Psychol Med. 2012;42(10):2015–2026. doi: 10.1017/S0033291712000128. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. International Journal of Psychiatry in Medicine. 2011;41(1):15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev. 2009;3:CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- 24.GOLD Scientific Committee, author. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. GOLD Scientific Committee; http://www.goldcopd.org/, Retrieved on 12/01/2006. [Google Scholar]
- 25.Beck A, Ward C, Mendelson M. Beck depression inventory (BDI) Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine, author. Guidelines for graded exercise testing and exercise prescription. Philadelphia: Lea & Febiger; 2005. [Google Scholar]
- 27.Kochanek K, Xu J, Minino A. Deaths preliminary data for 2009. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 28.NHLBI Morbidity and Mortality Chart book. [August 26, 2011]. Available at: http://www.nhlbi.nih.gov/resources/docs/chtbookhtm.
- 29.Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg S, Kalhan R. Biomarkers in chronic obstructive pulmonary disease. Translational Research. 2012;159(4):228–237. doi: 10.1016/j.trsl.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Wang D, Bai C, Wang X. Proteomics-based biomarkers in chronic obstructive pulmonary disease. J Proteome Res. 2010;9:2798–808. doi: 10.1021/pr100063r. PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Fattouh M, Alkady O. Inflammatory biomarkers in chronic obstructive pulmonary disease. Egyptian Journal of Chest Diseases and Tuberculosis. 2014;63:799–804. [Google Scholar]
- 33.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a metaanalysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Dekker M, Lee S, Hudson R, Kilpatrick K, Graham T, Ross R. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56(3):332–338. doi: 10.1016/j.metabol.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Mikkelsen U, Couppé C, Karlsen A, Grosset J, Schjerling P, Mackey A, Klausen H, Magnusson S, Kjær M. Life-long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev. 2013;134(11–12):531–540. doi: 10.1016/j.mad.2013.11.004. PubMed. [DOI] [PubMed] [Google Scholar]
- 38.Sugawara K, Takahashi H, Kashiwagura T, Yamada K, Yanagida S, Homma M, Dairiki K, Sasaki H, Kawagoshi A, Satake M, Shioya T. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respiratory Medicine. 2012;106:1526–1534. doi: 10.1016/j.rmed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 39.You T, Berman D, Ryan A, Nicklas B. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89(4):1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 40.Larsen A, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol. 2001;88(7):805–858. doi: 10.1016/s0002-9149(01)01859-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 41.Giannopoulou I, Fernhall B, Carhart R, Weinstock R, Baynard T, Figueroa A, Kanaley J. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Troseid M, Lappegard KT, Mollnes T, Arnesen H, Seljeflot I. The effect of exercise on serum levels of interleukin-18 and components of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7(6):579–584. doi: 10.1089/met.2009.0003. [DOI] [PubMed] [Google Scholar]
- 43.Lakka T, Lakka H, Rankinen T, Leon A, Rao D, Skinner J, Wilmore J, Bouchard C. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur Heart J. 2005;26:2018–2025. doi: 10.1093/eurheartj/ehi394. PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Beavers K, Brinkley T, Nicklas B. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11–12):785–793. doi: 10.1016/j.cca.2010.02.069. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheele C, Nielsen S, Pedersen B. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009;20:95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Smith J, Dykes R, Douglas J, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. PubMed. [DOI] [PubMed] [Google Scholar]
- 47.Timmerman K, Flynn M, Coen P, Markofski M, Pence B. Exercise training induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the antiinflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. PubMed. [DOI] [PubMed] [Google Scholar]
- 48.Stewart L, Flynn M, Campbell W, Craig B, Robinson J, McFarlin B, Timmerman K, Coen P, Felker J, Talbert E. Influence of exercise training and age on CD14+ cellsurface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. PubMed. [DOI] [PubMed] [Google Scholar]
- 49.Apolzan J, Flynn M, McFarlin B, Campbell W. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. 2006;61:388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 50.Gleeson M, McFarlin B, Flynn M. Exercise and tolllike receptors. Exerc Immunol Rev. 2006;12:34–53. PubMed. [PubMed] [Google Scholar]
- 51.Sloan R, Shapiro P, Demeersman R, McKinley P, Tracey K, Slavov I, Fang Y, Flood P. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103:1007–1011. doi: 10.1152/japplphysiol.00147.2007. PubMed. [DOI] [PubMed] [Google Scholar]
- 52.Garrod R, Ansley P, Canavan J, Jewell A. Exercise and the inflammatory response in chronic obstructive pulmonary disease (COPD)--Does training confer antiinflammatory properties in COPD? Med Hypotheses. 2007;68(2):291–298. doi: 10.1016/j.mehy.2006.07.028. PubMed. [DOI] [PubMed] [Google Scholar]
- 53.Woods J, Vieira V, Keylock K. Exercise, inflammation, and innate immunity. Neurol Clin. 2006;24(3):585–599. doi: 10.1016/j.ncl.2006.03.008. PubMed. [DOI] [PubMed] [Google Scholar]
- 54.Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J. Moderate exerciseimproves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005–1011. doi: 10.1016/j.jpsychires.2011.02.005. PubMed. [DOI] [PubMed] [Google Scholar]
- 55.Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, McKee DC, Reasoner J, Williams RS. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J Gerontol A Biol Sci Med Sci. 1989;44:M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 56.McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6:487–488. doi: 10.1037//0882-7974.6.3.487. PubMed. [DOI] [PubMed] [Google Scholar]
- 57.Blumenthal JA, Babyak MA, Carney RM, Huber M, Saab PG, Burg MM, Sheps D, Powell L, Taylor CB, Kaufmann PG. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc. 2004:746–755. doi: 10.1249/01.mss.0000125997.63493.13. [DOI] [PubMed] [Google Scholar]
- 58.Conn V. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Ann Behav Med. 2010;39(2):128–138. doi: 10.1007/s12160-010-9172-x. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Craft L. Exercise and clinical depression: examining twopsychological mechanisms. Psychology of Sport and Exercise. 2005;6(2):151–171. [Google Scholar]
- 60.Ossip-Klein DJ, Doyne EJ, Bowman ED, Osborn KM, Mcdougall-Wilson IB, Neimeyer RA. Effects of running or weight lifting on self-concept in clinically depressed women. Journal of Consulting and Clinical Psychology. 1989;57:158–161. doi: 10.1037//0022-006x.57.1.158. [DOI] [PubMed] [Google Scholar]
- 61.Foley LS, Prapavessis H, Osuch EA, De Pace JA, Murphy BA, Podolinsky NJ. An examination of potential mechanisms for exercise as a treatment for depression: a pilot study. Mental Health and Physical Activity. 2008;1:69–73. [Google Scholar]
- 62.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20:95–101. doi: 10.1093/sleep/20.2.95. PubMed. [DOI] [PubMed] [Google Scholar]
- 63.Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stressinduced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 64.Wager-Smith K, Markou A. Depression: a repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neurosci Biobehav Rev. 2011;35(3):742–764. doi: 10.1016/j.neubiorev.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capuro L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.García-Bueno B, Caso JR, Leza JC. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Neurosci Biobehav Rev. 2008;32(6):1136–1151. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 67.García-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008;32(6):1136–1151. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Dishman R, O'Connor P. Lessons in exercise neurobiology: the case of endorphins. Mental Health and Physical Activity. 2009;2:4–9. [Google Scholar]
- 69.Broocks A, Meyer T, Opitz M, Bartmann U, Hillmer-Vogek U, George A, Pekrun G, Wedekind D, Ruther E, Bandelow B. 5-HT1A responsivity in patients with panic disorder before and after treatment with aerobic exercise, clomipramine or placebo. European Neuropsychopharmacology. 2003;13:153–164. doi: 10.1016/s0924-977x(02)00177-3. [DOI] [PubMed] [Google Scholar]
- 70.Ernst C, Olson A, Pinel J, Lam R, Christie B. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? Journal of Psychiatry & Neuroscience. 2006;31:84–92. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 71.Lucassen P, Meerlo P, Naylor A, Van Dam A, Dayer A, Fuchs E, Oomen C, Czeh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. European Neuropsychopharmacology. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]