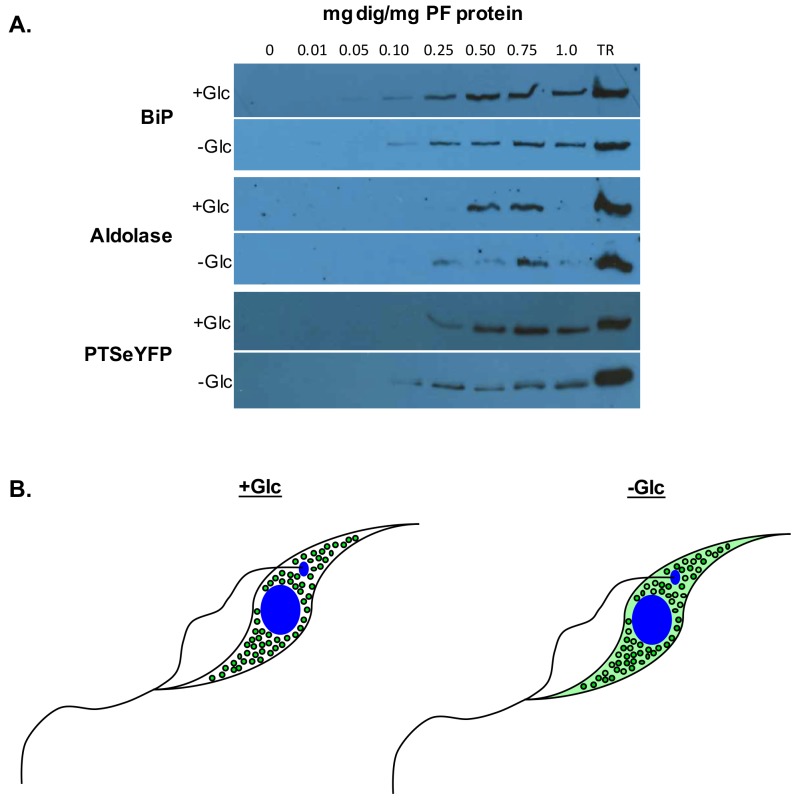
Fig 3. Glycosomal matrix protein import is more tightly regulated when cultured in glucose rich media.
(A) Digitonin fractionations of PF cells grown in high glucose (+Glc) and low glucose (-Glc) reveal that glycosomal proteins are mislocalized under low-glucose conditions. Digitonin binds to membrane cholesterol. At lower concentrations, the plasma membrane is solubilized. Higher concentrations are required to dissolve intracellular membranes. Using this protocol, cytoplasmic proteins are released at concentrations below 0.05 mg dig/mg PF protein [74]. Lysates were treated with increasing concentrations of digitonin and intact organelles recovered by centrifugation. Pelleted samples were analyzed by western blotting with antibodies against an ER marker (BiP), a glycosomal matrix protein (aldolase), and a glycosomal reporter protein consisting of a peroxisomal targeting signal (PTS2) fused to an eYFP (PTSeYFP). While BiP is released at 0.10 mg dig/mg PF protein under both conditions, aldolase and PTSeYFP are released at lower concentrations of digitonin under -Glc conditions when compared to +Glc conditions. Aldolase is released at 0.50 mg dig/mg PF protein in +Glc conditions and 0.25 mg dig/mg PF protein in cells grown in -Glc condition. Similarly, PTSeYFP is released at 0.25 mg dig/mg PF protein in cells cultured in +Glc conditions and 0.10 mg dig/mg PF protein under -Glc conditions. The difference in glycosomal protein release and not the control ER resident protein BiP suggests that mislocalized glycosomal proteins can be detected extraglycosomally under low-glucose conditions. (B) Model of glycosome protein mislocalization in low glucose. In high glucose, glycosomal proteins localize exclusively to glycosomes, and mislocalization of any glycosomal proteins (or possibly a particular subset of glycosomal proteins) may be lethal to PF cells. Under low-glucose conditions, mislocalization of glycosomal matrix proteins is more tolerated [12,13], and matrix proteins can be detected in the cytoplasm prior to import. Glycosomal proteins depicted in green.