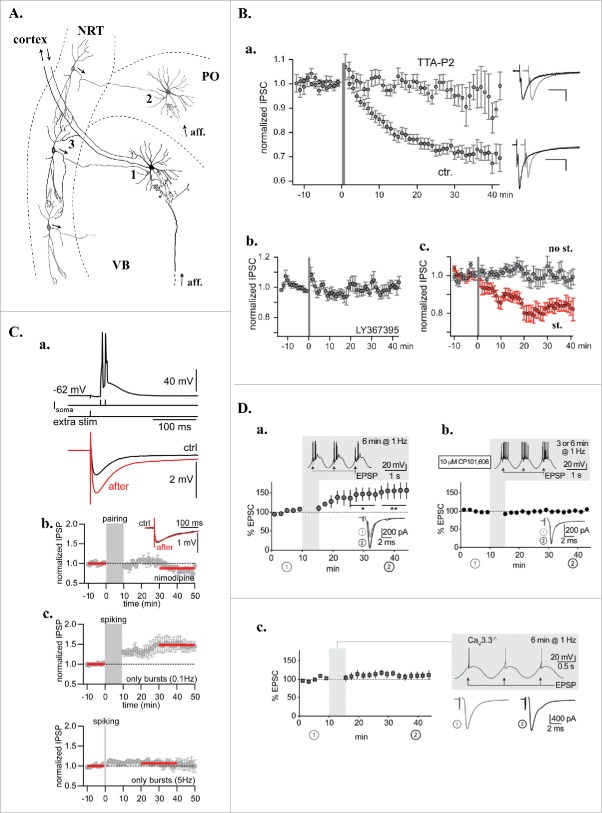
Figure 1.
T-dependent plasticity in the intra-thalamic network. A. Schematic drawing of the thalamic network presenting 3 synapses (1,2,3) where long-term plasticity mechanisms were described. VB: ventrobasal nucleus; PO: posterior medial nucleus; NRT: nucleus reticularis thalami; aff.: extra-thalamic afferences. B. Homo and hetero synaptic LTD of the rat NRT-VB GABAergic synapse (synapse 1 in A). a. Mean normalized inhibitory current amplitude evoked in thalamocortical neurons by NRT fiber stimulation. At time 0 (gray bar) short series of periodic (1.6 Hz) high-frequency stimulations (200 Hz) were applied to the NRT afferences while depolarizing the postsynaptic thalamocortical neurons from −80 to −30 mV to mimic both the incoming bursting activity of the NRT fibers and the concomitant LTS-associated depolarizations of the thalamocortical neurons that occur during sleep slow waves. Following the induction protocol an LTD of the GABAergic synapses developed in control condition (ctr., n = 22) but was not induced in the presence of the T-type current antagonist, TTA-P2 (3µM, n = 10). Examples of inhibitory currents evoked before (black traces) and after (gray traces) the induction protocol in both conditions are presented on the right. b. Demonstrating its heterosynaptic nature, LTD was suppressed in the presence of LY-367385 (100 µM, n = 11), a selective antagonist of the type 1 mGluRs that are expressed post-synaptically at the cortico-thalamic synapses .c. Mean normalized inhibitory current amplitudes evoked by stimulating 2 independent NRT pathways converging on the same thalamocortical neurons. LTD was only observed in the NRT pathway stimulated during the induction protocols (red dots) confirming the homosynaptic nature of the plasticity mechanism (n = 11). C. LTP of GABAergic synapses in rat thalamic posterior median thalamus (synapse 2 in A). a. top - induction protocol: Two current steps (100 Hz) injected in the thalamocortical neurons to evoke action potentials on top of a low-threshold Ca2+ spike were paired with an inhibitory post-synaptic potential (IPSP) triggered by extracellular stimulation. Bottom: Averaged IPSPs during baseline (ctrl, black) and after LTP induction (after, red). b. Normalized and averaged IPSP amplitudes over time demonstrating that LTP did not develop in the continuous presence of the L-type Ca2+ channel antagonist, nimodipine (10µM; n = 5). c. Normalized and averaged IPSP amplitudes over time showing that LTP can be induced with postsynaptic stimulation alone (bursting rhythmicity 0.1Hz, n = 7) in the absence of presynaptic stimulation. The LTP was reduced upon increasing the bursting rhythmicity and disappeared at frequencies higher than 5 Hz (n = 7). In b. and c., red bars indicate IPSPs used to calculate the amplitude ratio between control and post-induction protocol periods. D. Burst discharge via Cav3.3 channels induced LTP at thalamoreticular inputs (synapse 3 in A) when coactivated with GluN2B-containing NMDA receptors. a. Normalized and averaged excitatory post-synaptic currents (EPSCs) evoked in NRT neurons during baseline and after pairing synaptic input with LTS mediated bursting activities for 6 min (see shadowed inset). The pairing protocol induced a significant potentiation of the EPSCs (*p < 0.05, **p < 0.01; n = 7). Traces in the lower insets are average EPSCs evoked during baseline (1, gray) and during the last 10 min of recording (2, black). b. LTP was prevented in the presence of the GluN2B-NMDA receptor antagonist CP101,606 (n = 9). c. In Cav3.3−/− KO mice where low-threshold bursting is largely suppressed, pairing synaptic input with action potential firing elicited by sinusoidal current injection did not allow LTP induction (n = 8). Modified with permission from: (B),45 (C),46 (D).72.