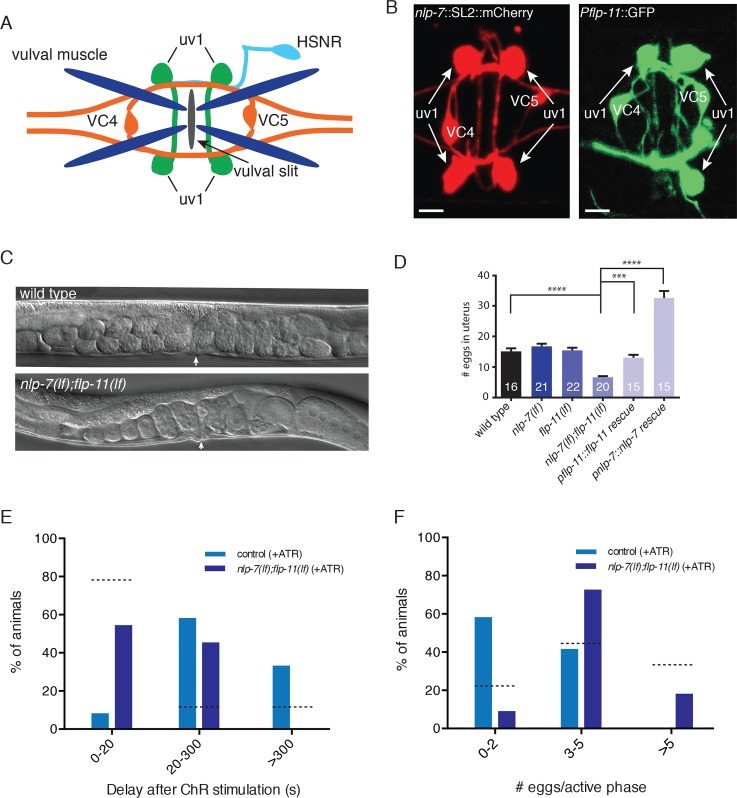
Fig 3. NLP-7 and FLP-11 are expressed in uv1 cells and act synergistically to inhibit egg-laying.
(A) Schematic of egg-laying neuromusculature. (B) Fluorescent images of adult hermaphrodites expressing either Pnlp-7::NLP-7::SL2::mCherry or Pflp-11::GFP [36] reporter transgenes. In addition to the egg-laying circuit, both reporters label head and tail neurons (see S1 and S2 Figs). Scale bars, 5 μm. (C) Representative DIC images of wild type (upper) and nlp-7(lf);flp-11(lf) double mutants (lower). Arrows indicate position of the vulva. (D) Quantification of eggs in utero. Bars represent mean ± SEM for each genotype. Numbers in bars indicate the n for each group. **** p<0.0001, *** p<0.001 ANOVA with Sidak’s post-hoc test. In this and all subsequent figures, nlp-7(lf) and flp-11(lf) refer to the tm2984 and tm2709 alleles respectively. (E) Time interval between light stimulus and subsequent egg-laying event. For E and F, light stimulation (5 s, 100 W/m2) of animals stably expressing uv1::ChR2 was initiated immediately after the first egg-laying event of an active phase. Percent of animals that perform a second egg laying event within 20 s, 20–300 s, or after >300 s is indicated. control, n = 12; nlp-7;flp-11, n = 11. p<0.0001, Chi-square test. Dashed lines indicate percent of animals in each category for control stimulation in the absence of retinal (from Fig 2). (F) Quantification of total egg-laying events following the initial event within an active phase. Percent of animals laying 0–2, 3–5, or >5 eggs following uv1 photostimulation is shown. Dashed lines indicated percent of animals in each category for control stimulation in the absence of retinal (from Fig 2). ATR, exogenous retinal. control, n = 12; nlp-7;flp-11, n = 11. p<0.0001, Chi-square test.