Figure 5. YAP abolishes TBK1 activity through its C-terminal transactivation domain.
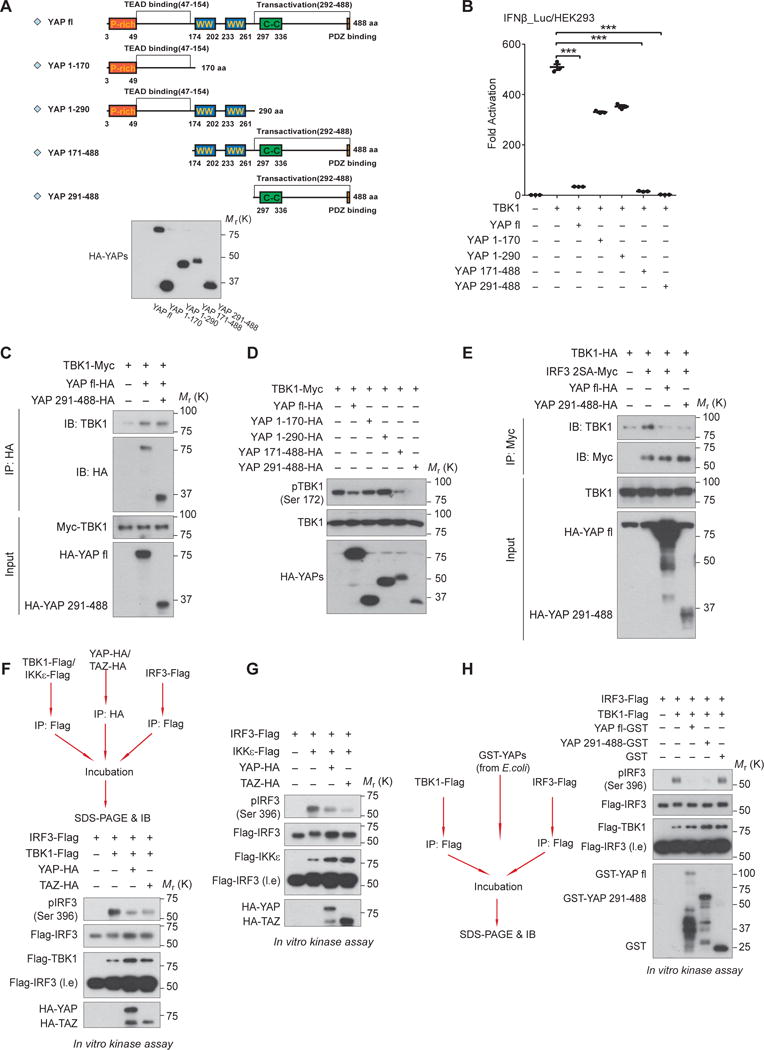
(A and B), Serial truncations of YAP were generated as depicted (A, upper panel) and their expressions were verified by immunoblotting (A, lower panel). Effects of the full-length or YAP truncations on antiviral signaling were measured by IRF3 transactivation, which revealed a marked inhibition by YAP transactivation domain (a.a. 291 – 488) (B). n=3 independent experiments. Mean ± SEM. ***P<0.001, by ANOVA test and Bonferroni correction. (C and D), Similar to the full-length protein, YAP transactivation domain was sufficient to interact with TBK1 (C) and to block TBK1 activation (D), assessed by co-immunoprecipitation and by immunoblotting of TBK1 Ser172 phosphorylation, respectively. (E), Interaction of TBK1 and IRF3 was severely interrupted in the presence of YAP transactivation domain, revealed by co-immunoprecipitation. (F and G), Addition of either YAP or TAZ separately purified from HEK293T cells in the in vitro kinase assays suppressed the catalytic activity of TBK1 (F) or IKKε (G) on the substrate IRF3. (H), Likewise, addition of GST-tagged full-length or transactivation domain of YAP that was expressed and purified from E.coli blocked TBK1-mediated IRF3 phosphorylation, in an in vitro kinase assay. Unprocessed images of blots are shown in Supplementary Figure 6. Statistics source data are provided in Supplementary Table 1.
