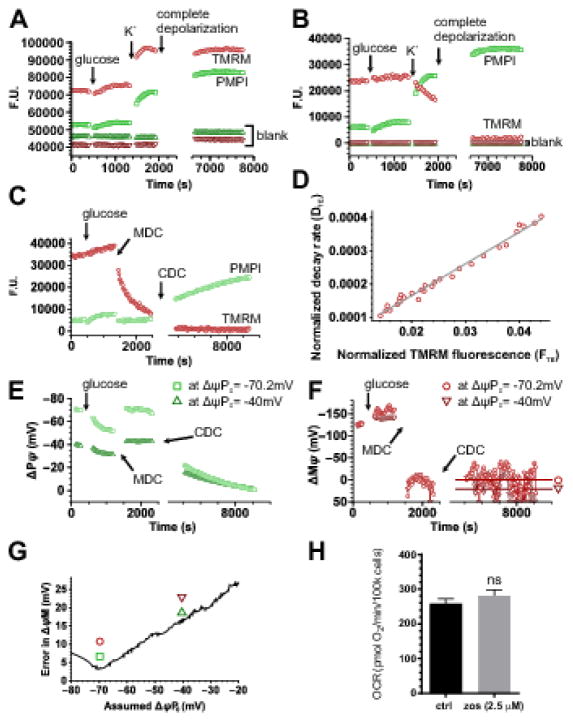
Fig. 2. Calibration of ΔψM and ΔψP using a fluorescence microplate reader.
(A–B) Spectral unmixing. (A) Unprocessed recording shown as fluorescence units in a well containing INS-1 832/13 cells and in a blank well. To demonstrate spectral crossbleed, cells were exposed to glucose (10 mM) and high extracellular [K+] (64 mM) increasing PMPI fluorescence and decreasing TMRM fluorescence. (B) Fluorescence intensities of the data in A after spectral unmixing (see Error! Reference source not found.) and blank well background subtraction. (C) Spectrally unmixed and background subtracted fluorescence recording for the determination of ΔψP0, ΔψM0 and kT. The calibration is based on the decay of TMRM fluorescence after acute and complete mitochondrial depolarization provided by MDC, followed by complete depolarization of the cell by CDC (see compositions in Error! Reference source not found.). Data are from a representative single well chosen from 16 technical replicates in each of 10 independent experiments. (D) Linear regression analysis to calculate ΔψM0 and kT as described in Error! Reference source not found., corresponding to TMRM fluorescence intensities measured between MDC and CDC additions in panel C. The good linear fit indicates that the experimental system followed the biophysical model assuming a time- and ΔψP-dependent leakage of TMRM from the cytosol to the extracellular space. (E) Calibrated ΔψP and (F) calibrated ΔψM corresponding to panel C. ΔψP0 was numerically optimized (resulting in a ΔψP time course for each iteration, see E) to give minimal deviation from 0 mV in calibrated ΔψM at the end of the recording, indicated by horizontal lines in F. Circles and squares indicate potentials calibrated at the optimum ΔψP0, while triangles indicate those at an arbitrary different ΔψP0 (−40 mV). (G) Dependence of the deviation of mean calibrated ΔψM from 0 mV at the end of the recording as a function of assumed ΔψP0. Symbols correspond to panels E–F and indicate the ΔψP0 used for the calibration of traces. (H) Zosuquidar (zos) used to prevent pumping of fluorescent probes during the assay does not alter basal respiration. INS-1 832/13 cells were preincubated in 2.5 μM zosuquidar for 90 min before cell respirometry. ns, not significant.