Fig. 3.
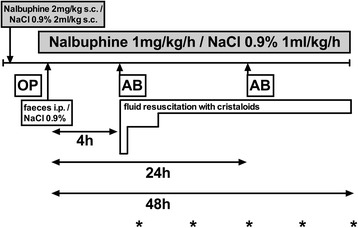
Design of long-term experiments. Animals underwent either faecal peritonitis or sham (NaCl 0.9%) i.p. injection and were subsequently randomized to either nalbuphine (1 mg/kg/h) or placebo (NaCl 0.9% 1 ml/kg/h) infusion. Asterisks indicate frequency of clinical scoring (6, 12, 24, 36, and 48 h). AB: antibiotics (ceftriaxone, 30 mg/kg i.v.); OP operation (=instrumentation). The subcutaneous administration of nalbuphine in combination with lidocaine was requested by the animal ethics committee in order to avoid post-operative pain after termination of isoflurane anaesthesia. Placebo animals received a subcutaneous injection of normal saline (2 ml/kg)
