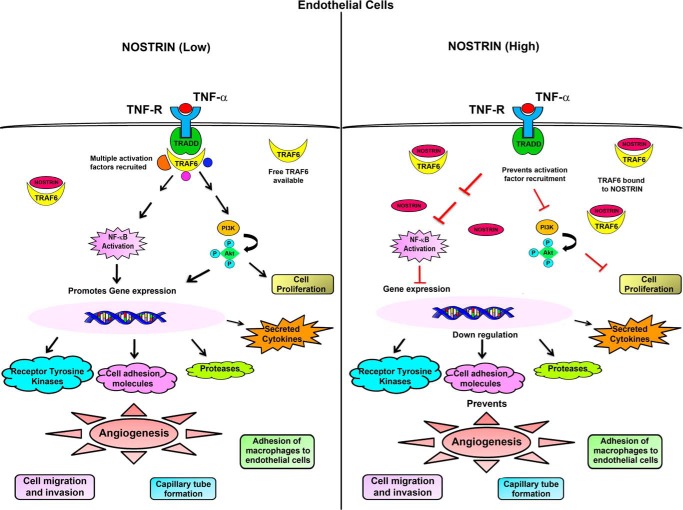
Figure 9.
A schematic representation or summary of the alteration in endothelial cell signaling by aberrant NOSTRIN expression. Binding of TNF-α to its cognate receptor leads to the recruitment of several adaptor proteins and activation factors that in turn can activate the NFκB pathway and also promote phosphorylation of AKT. When NOSTRIN levels are low, free TRAF6 molecules are available to bind to TRADD and transduce the signal to downstream activation factors. Rapid activation of the NFκB pathway leads to expression of several pro-angiogenic genes along with production of pro-inflammatory cytokines that are direct targets of the transcription factor NFκB. On the other hand, when NOSTRIN levels are very high, NOSTRIN can bind directly to TRAF6 and thus inhibit its binding to TRADD on TNFα stimulation. This is followed by suppression of NFκB activity along with inhibition of AKT phosphorylation. As a consequence, gene expression is inhibited, and multiple functions of endothelial cells required for angiogenesis are severely compromised.