Figure 6.
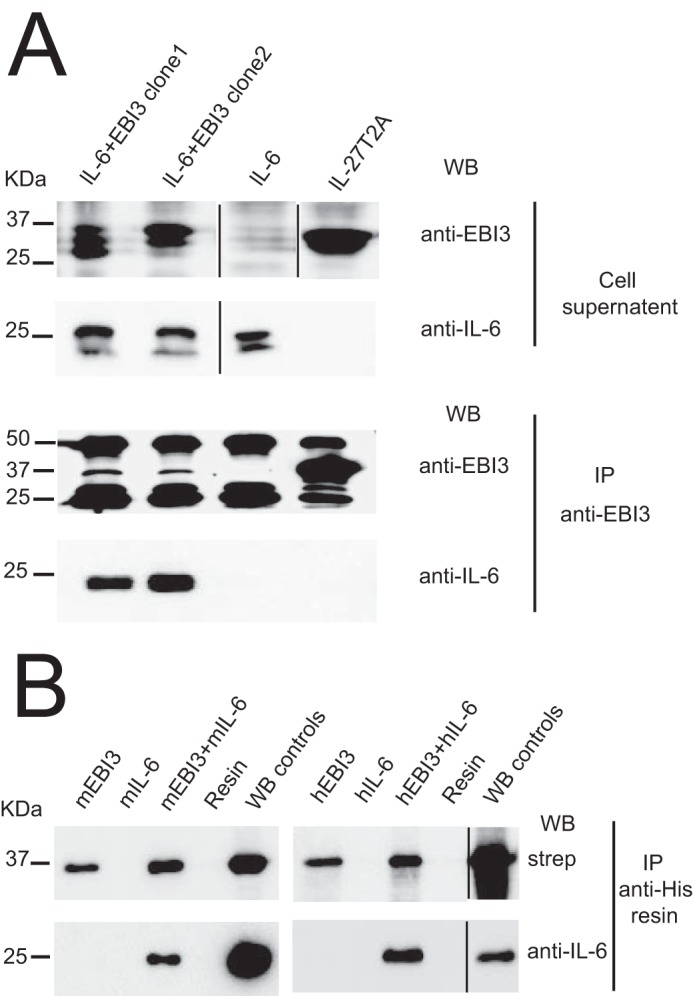
EBI3 forms a secreted complex with IL-6. A, HEK-239 Flp-InTM cells were transfected with expression vectors coding for mEBI3-T2A-IL-6 (clone nos. 1 and 2), mIL-27T2A, and mIL-6. Proteins were immunoprecipitated (IP) from cellular supernatants using anti-mEBI3 mAb and anti-rat IgG-agarose resin. Signals for mEBI3 (35 kDa) and mIL-6 (25 kDa) were revealed by successive incubation with anti-mEBI3 and biotinylated anti-mIL-6 Abs followed by anti-rat IgG-HRP and streptavidin-HRP, respectively. Signals at 50 and 25 kDa represent background because of the detection of the Ig heavy and light chains eluted from rat IgG-agarose by the secondary antibody used for EBI3 detection. B, His-biotin-tagged recombinant mouse or human EBI3 and unconjugated mouse or human IL-6 were subjected to IMAC. Nickel-nitrilotriacetic acid (Ni-NTA) resin incubated without the addition of EBI3 and IL-6 was used as negative control (resin lanes). EBI3 (100 ng) and IL-6 (20 ng) were used as positive detection controls. Signals were revealed with either streptavidin-HRP or anti-IL-6 followed by HRP-conjugated anti-IgG. WB, Western blotting.
