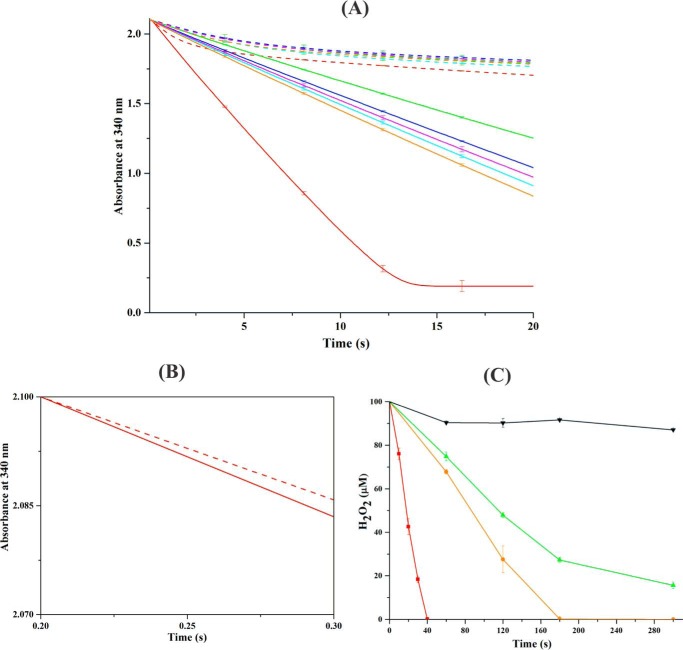
Figure 6.
Peroxidase activity of the AhpF-AhpC complex. A, NADH-dependent peroxidase activity was measured for 0.4 μm WT EcAhpF (red) or the different linker mutants, EcAhpF(E200A) (cyan), EcAhpF(K201A) (magenta), EcAhpF(R202A) (orange), EcAhpF(K201A/R202A) (blue), and EcAhpF(E200A/K201A/R202A) (green) in the presence of 30 μm EcAhpC, 300 μm NADH with (straight lines) or without (dashed lines) 1 mm H2O2 in a reaction mixture containing 50 mm phosphate buffer, pH 7.0, 100 mm ammonium sulfate, and 0.5 mm EDTA. B, the initial rate of NADH oxidation for WT EcAhpF and EcAhpC with (straight lines) or without (dashed lines) 1 mm H2O2. C, to determine the H2O2 decomposition, 0.4 μm (■) WT EcAhpF, (●) EcAhpF(R202A), and (▴) EcAhpF(E200A/K201A/R202A) were added to the reaction mixture containing 30 μm EcAhpC, 750 μm NADH, and 100 μm H2O2. The residual H2O2 in the reaction mixtures was measured at the indicated times using ferrous oxidation xylenol reagent. The control was measured in the absence of EcAhpF (▾). The linker mutants significantly affect the EcAhpC-dependent peroxidase activity. The data presented were averaged of three independent measurements, and error bars represent the standard deviation.