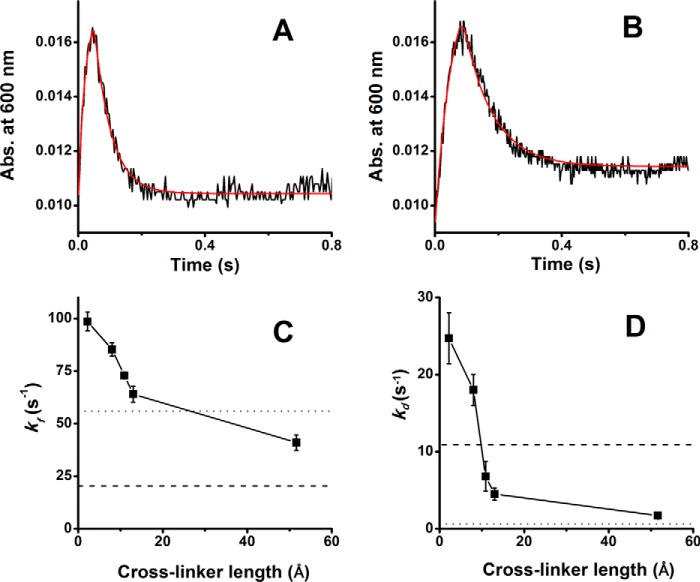
Figure 7.
Effect of domain cross-linking on the kinetics of flavin semiquinone formation and disappearance. Fully oxidized CLSS whose domains were either free (DTT-treated) or were cross-linked with the various bismaleimides was mixed with excess NADPH at 10 °C, and flavin semiquinone was monitored at 600 nm versus time. A and B, absorbance traces for the disulfide cross-linked or free CLSS, respectively, with the lines of best fit indicated in red. C and D, rates of flavin semiquione formation and decay derived from the lines of best fit, respectively, for CLSS cross-linked by a disulfide bond or with the bismaleimides of the indicated length. The dashed and dotted lines indicate the rates of flavin semiquinone formation and decay obtained for the free CLSS and for nNOSr, respectively, under the same conditions. Data are the mean ± S.D. for 5–7 trials.