Abstract
Study design:
Experimental study.
Objectives:
To investigate a modified compression model of spinal cord injury (SCI) in adult rats by using a room-air- inflated Fogarty balloon catheter.
Setting:
Kaohsiung, Taiwan.
Methods:
The rats were divided into injury, sham-operated and control groups. A 2-French Fogarty catheter was passed from the lumbar spine (L3–L4) epidurally, with a mini-laminectomy under the microscope, to the level of thoracic spine (T6–T7). The actual site of the catheter tip was confirmed with X-ray. The balloon of Fogarty catheter then was inflated with room air, 0.2 ml, for 10 min. Mini-laminectomy was performed without inserting the catheter in the sham-operated group. Quantitative neurological outcomes were evaluated with the Basso, Beattie and Bresnahan (BBB) locomotor rating scale daily. The gene expression of nitric oxide synthases (NOSs) of the spinal cord was investigated at the end of the functional assessment.
Results:
The mean BBB locomotor scores were 10±1.85 and 10±1.85, respectively, on days 1 and 3 in the injury group, and 21 and 20.29±0.69, respectively, in the sham-operated group. There was a significantly increased gene expression of inducible NOS in the SCI group compared with the sham-operated group and control group. Endothelial NOS gene expression was not significantly different among the groups.
Conclusion:
The functional and molecular assessments show that this modified balloon-compression technique is a reproducible, simple and inexpensive model of SCI in rats.
Introduction
Experimental models of spinal cord injury (SCI) have increased our understanding of the pathophysiology and provide an opportunity to search therapeutic strategies in vivo. Contusion with weight drop, mechanical displacement with electromagnetic device, compression with aneurysm clip, cutting injury with complete/partial transection and compression model with balloon inflation1, 2, 3 are the mentioned and established models. Most of the models need detailed setting, and such studies are laborious and require expensive equipment.2 Epidural compression with the Fogarty catheter balloon is an easier, less-expensive and reproducible model.1, 4 However, a microfluidic pump is needed. The purpose of this study was to modify the catheter balloon-compression model without the use of a micropump. Quantitative functional outcomes were evaluated. Furthermore, the gene expression of NOSs of the spinal cord was investigated as a molecular assessment of the modified model.
Materials and methods
This experimental animal study was approved by the Laboratory Animal Center, Kaohsiung Medical University, Kaohsiung, Taiwan. Twenty-one male Sprague–Dawley rats (Taiwan National Laboratory Animal Center, Taipei, Taiwan), with weight 300–350 g, were randomized into three groups: SCI, sham-operated and healthy control groups.
SCI and sham operation
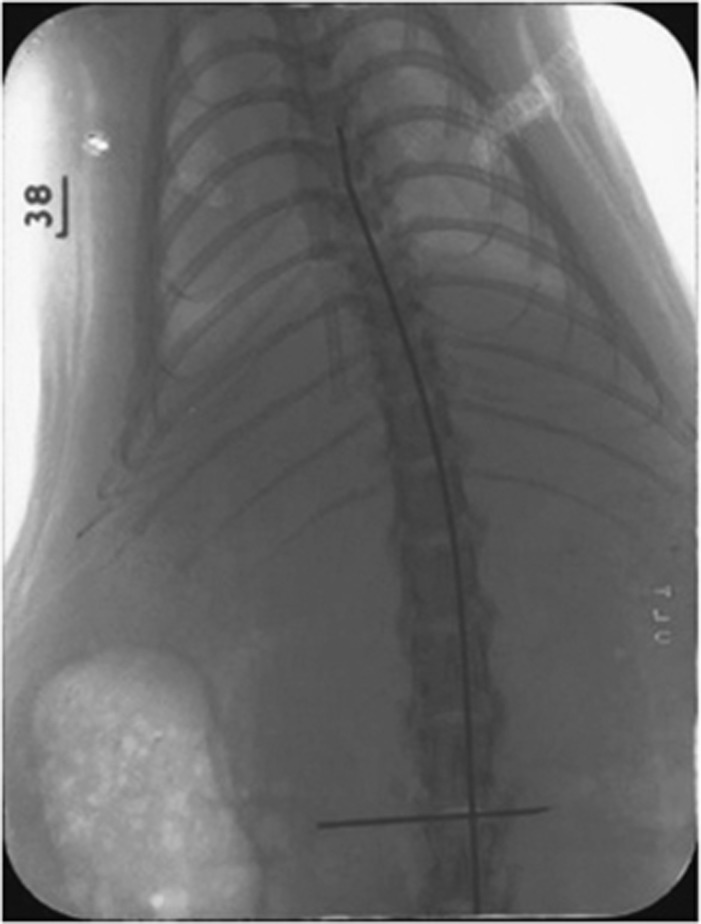
The animals were anesthetized with intraperitoneal injection of 3% sodium pentobarbital (20 mg kg−1). The rats were placed and fixed in a prone position. A linear incision was made at the level of lumbar spine, L3–L4. Mini-laminectomy was performed with a microscope. The 2-French Fogarty Catheter (Baxter, Irvine, CA, USA) was passed epidurally up to the level of thoracic spine, T6–T7. The actual position of the catheter tip was confirmed with X-ray immediately (Figure 1). The experiment proceeded only when the tip of the catheter was at the desired level. The balloon of the Fogarty catheter was then inflated with room air, 0.2 ml, with a 1-ml syringe for 10 min, rather than with saline and micropump.1 Mini-laminectomy was performed without inserting the catheter in the sham-operated group.
Figure 1.
The X-ray after the Fogarty catheter is inserted. The tip of the catheter is at the level of T6–T7. The needle shows the catheter inserted at the level of L3–L4.
Functional assessment of neurological deficits
Quantitative neurological outcomes were evaluated on the day before, day 1 and day 3 after the experimental procedure, with the Basso, Beattie and Bresnahan rating scale established and described before.5, 6 The consequences of the injury were recorded with the 21-point open-field locomotion scores.
Isolation of RNA in spinal cord tissue and detection of nitric oxide synthase mRNA expression using semiquantitative reverse transcription-PCR
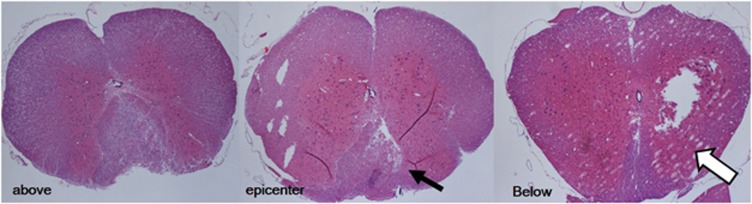
The gene expression of nitric oxide synthases of the spinal cord was investigated at the end of functional assessment, that is, day 3 after the experimental procedure. Animals were killed via the intracardial perfusion with saline solution. Figure 2 shows the representative horizontal cross-sections of the spinal cord at 1 cm above, epicenter of and 1 cm below the level of the balloon-compression injury with hematoxylin and eosin stain. Spinal cord 1 cm long, centering on the level of T7, was harvested and frozen in liquid nitrogen, and pulverized with a pestle in a mortar immediately. Total RNA was isolated using a SV Total RNA Isolation System (Promega, Madison, WI, USA). The integrity of the isolated RNA was confirmed by agarose gel electrophoresis. The RNA concentration was determined by spectrophotometry. For reverse transcription, samples of 1 μg total RNA were reverse transcribed into cDNA in 20 μl of reaction mixtures containing 200 units of Superscript II (Gibco BRL, Rockville, MD, USA), 0.5 μg oligo(dT)12–18, 200 pmol dithiothreitol, 10 pmol dNTP and 40 units of ribonuclease inhibitor (Gibco BRL, Life Technologies, Rockville, MD, USA) in a buffer supplied with the enzymes. The reverse transcription procedure was performed according to the manufacturer's protocol (Superscript II; Gibco BRL, Rockville, MD, USA). The resulting cDNA was frozen at −20 °C for mRNA detection. The target genes were amplified by PCR using a Taq DNA Polymerase Kit (FastStart Taq DNA Polymerase; Roche Diagnostics GmbH, Mannheim, Germany). A volume of 1.5 μl cDNA from reverse transcription was replicated in PCR reactions in a total volume of 25 μl containing Taq DNA Polymerase (0.63 units), buffer supplied with enzymes, MgCl2 (50 pmol), dNTP (200 μmol) and primers (20 pmol). Duplex PCR was performed using primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) gene. The PCR conditions were 94 °C for 1 min followed by 30 cycles of 60 °C (GAPDH and iNOS) or 69 °C (eNOS) for 30 s, and extension at 72 °C for 1 min. The primer sequences of GAPDH, iNOS and eNOS were designed via a commercial computer software (Lightcycler Probe Design; Roche Diagnostics GmbH). All primers were made by the Gibco BRL. Five microliters of the reaction products of each transcript were mixed with 1 μl loading dye, and the mixtures were then electrophoresed on a 1.5 or 2.0% agarose gel containing ethidium bromide (0.1 μg ml−1). After electrophoresis, the gels were visualized by ultraviolet-induced fluorescence. The expected size for the DNA fragments of GAPDH, iNOS and eNOS was 219, 317 and 588 base pairs, respectively. The PCR products were quantified by a densitometer interfaced with Bioprofil Image Analysis Software (Vilber Lourmat, Marne-la-Vallée, France). The results were expressed as ratios relative to GAPDH.7
Figure 2.
The representative horizontal cross-sections of the spinal cord at 1cm above, epicenter of and 1cm below the level of balloon-compression injury, with hematoxylin and eosin stain. Cord damage and tissue loss can be observed over the dorsal and lateral sides of the spinal cord (arrows). Cavitations were also observed (hollow arrow).
Statistical analysis
All the functional assessment data are expressed as the mean±s.e.m. Gene expression comparison between groups was performed with the nonparametric Kruskal–Wallis test. Significant differences were considered as the probability value was less than 0.05.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of the animals followed during the course of this research.
Results
SCI induced by the air-inflated balloon compression is functionally steady and reproducible
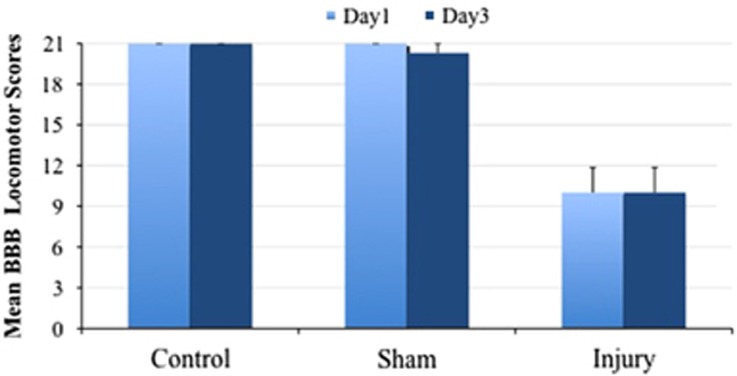
The functional assessments of the different groups of animals are summarized in Table 1 and Figure 3. The mean Basso, Beattie and Bresnahan locomotor scores in the group of SCI rats were 10±1.85 and 10±1.85 on days 1 and 3, respectively. The mean Basso, Beattie and Bresnahan scores of the sham-operated group were 21 and 20.29±0.69 on days 1 and 3, respectively. No rats in this group were paraplegic.
Table 1. Basso, Beattie and Bresnahan locomotor scores on days 1 and 3 after SCI.
| Group | Day 1 | Day 3 |
|---|---|---|
| SCI | 10±1.85* | 10±1.85* |
| Sham operation | 21 | 20.29±0.69 |
| Healthy control | 21 | 21 |
Abbreviation: SCI, spinal cord injury. *P<0.05, compared with sham operation and healthy control groups.
Figure 3.
Basso, Beattie and Bresnahan locomotor scores in different groups of rats on days 1 and 3 after SCI.
The mRNA expression of iNOS but not eNOS increases after SCI
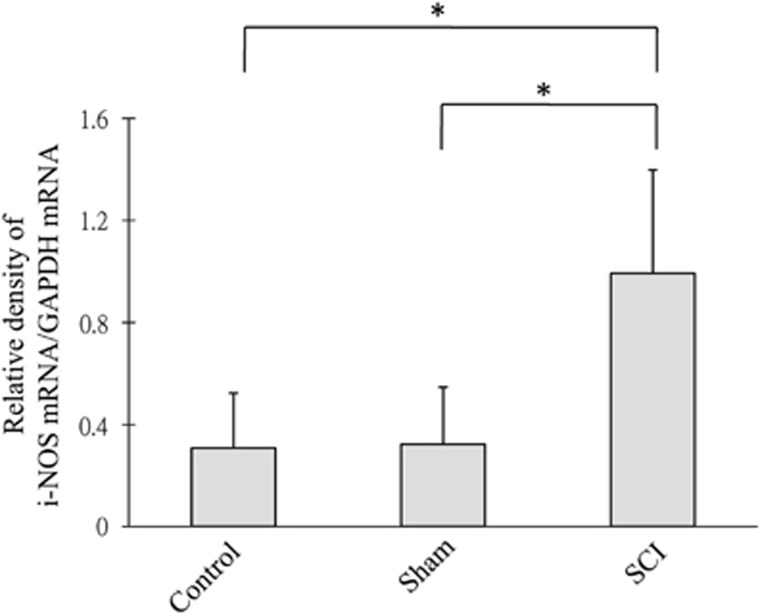
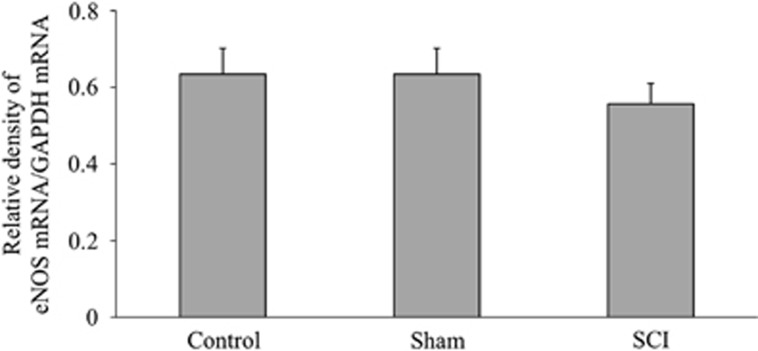
The SCI group showed significant increases in the levels of iNOS/GAPDH ratio as compared with both the healthy control and sham-operated groups (Figure 4). The results of eNOS/GAPDH were not significantly different among the healthy control, sham-operated and SCI groups (Figure 5).
Figure 4.
iNOS mRNA expression—the ratio of mRNA expression of iNOS relative to GAPDH among different groups is shown. *P<0.05 when SCI group is compared with sham-operated and healthy control groups.
Figure 5.
eNOS mRNA expression—the ratio of mRNA expression of eNOS relative to GAPDH among different groups are shown. There is no significant difference among the healthy control, sham-operated and SCI groups.
Discussion
With the steady functional and molecular assessment results, we presented a modified balloon-compression technique of SCI in rats, which is simple, reproducible and inexpensive.
SCI has a major impact on the individual both physically and psychologically. Although it is not a common cause of disability, with an estimated incidence of around 40 cases per million in the United States, the social and economic costs of SCI are not proportional to its frequency.8
Experimental models of SCI are critical for the development of therapeutic strategies to treat the clinical problems. Essentially the injury model should be consistent and have reproducible outcomes, anatomical and behavioral, even physiological or molecular. Many efforts have been made to integrate and standardize the animal models and experimental results.9 However, using a variety of different models is also encouraged to learn more about the regenerative capability and plasticity of different systems.3
The balloon-compression technique to produce SCI in adult rats is simple and reproducible, with the use of a fluidic micropump or micromanipulator.1, 10 In this study, the balloon was inflated with room air instead of using saline and a micropump. Functional assessment revealed that the compression induced a consistent injury. Some minor injuries/motor deficits were observed in the sham-operated group on day 3 (Figure 1 and Table 1) although a microscope was applied during the procedure. To carry out an optimal experiment, meticulous surgical skill is always required and the possibility of postoperative complications also should be considered, for example, delayed epidural hematoma compression or infection.
The rate and volume of balloon inflation are also factors considered during the procedure. A slow rate of balloon inflation prevents the development of neurogenic pulmonary edema.10 A volume of 0.2 ml of room air was applied in this study. Further studies designed with different inflation volume are needed to characterize the gradation of injury and to produce a submaximal injury that will be useful for studying the pathophysiology and effects of potential therapies.1
Nitric oxide (NO) is involved in a variety of physiological processes in the central nervous system, and its protective as well as detrimental effects are observed in several diseases states of the central nervous system, including SCI. Nitric oxide synthases generate NO by conversion of L-arginine to L-citrulline.11
iNOS, one of the three isoforms of nitric oxide synthase, has been noted to have a major role in the initiation/exacerbation of inflammatory/degenerative conditions in the CNS, such as SCI, ischemia, multiple sclerosis, Alzheimer's disease, and inherited peroxisomal and lysosomal disorders. Activation of iNOS and NO generation has been accepted as a marker and therapeutic target to these diseases.12 Mice without iNOS gene tended to have a better functional outcome than wild-type mice after SCI.13 In this study, the mRNA expression of iNOS significantly increased in the group of experimental SCI, as compared with the sham-operated and the healthy control groups. The result is compatible with the previous studies,14, 15, 16 that is, this modified balloon-compression technique is shown to be a reproducible and reliable procedure in the molecular analysis.
eNOS plays a neuroprotective role after cerebral ischemia and hemorrhage.7, 17, 18 However, there is insufficient and variable data about eNOS expression and activity after SCI.16, 19, 20, 21 In a study with rats, eNOS activity returned to a normal level 30 min after injury with a transient reduction. The change may be correlated with the reported vascular changes.16 The eNOS activity in young rats has been found to be relatively greater than that in adult rats after SCI, with a neural protective function.19 A study in mice suggested that severe SCI resulted in degraded eNOS, whereas mild SCI activated eNOS in the subacute stage.17 In this study, the mRNA expression of eNOS did not increase on day 3 after the experimental SCI, with a consistent result as compared with the sham-operated and healthy control groups. Further study focusing on the earlier expression/activity of eNOS may help to clarify the variety.
Conclusion
The functional and molecular assessments show that this modified balloon-compression technique is a reproducible, simple and inexpensive model of SCI in rats.
Data archiving
There were no data to deposit.
Acknowledgments
We acknowledge Susan Wan-Hsuan Chen for the specialized assistance to the statistical analysis and Dr Shih HC for the efforts in gene expression analysis. This study was supported by a grant from the Kaohsiung Medical University Hospital (94-KMUH-025).
Footnotes
The authors declare no conflict of interest.
References
- Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma 2001; 18: 1399–1407. [DOI] [PubMed] [Google Scholar]
- Farooque M. Spinal cord compression injury in the mouse: presentation of a model including assessment of motor dysfunction. Acta Neuropathol 2000; 100: 13–22. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Harper GP, Bradbury EJ. Progress in spinal cord research - a refined strategy for the International Spinal Research Trust. Spinal Cord 2000; 38: 449–472. [DOI] [PubMed] [Google Scholar]
- Marques SA, Garcez VF, Del Bel EA, Martinez AM. A simple, inexpensive and easily reproducible model of spinal cord injury in mice: morphological and functional assessment. J Neurosci Methods 2009; 177: 183–193. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12: 1–21. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996; 139: 244–256. [DOI] [PubMed] [Google Scholar]
- Shih HC, Lin CL, Wu SC, Kwan AL, Hong YR, Howng SL. Upregulation of estrogen receptor alpha and mediation of 17beta-estradiol vasoprotective effects via estrogen receptor alpha in basilar arteries in rats after experimental subarachnoid hemorrhage. J Neurosurg 2008; 109: 92–99. [DOI] [PubMed] [Google Scholar]
- Jackson AB, Dijkers M, Devivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil 2004; 85: 1740–1748. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma 1996; 13: 343–359. [DOI] [PubMed] [Google Scholar]
- Sedy J, Zicha J, Kunes J, Jendelova P, Sykova E. Rapid but not slow spinal cord compression elicits neurogenic pulmonary edema in the rat. Physiol Res 2009; 58: 269–277. [DOI] [PubMed] [Google Scholar]
- Conti A, Miscusi M, Cardali S, Germano A, Suzuki H, Cuzzocrea S et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev 2007; 54: 205–218. [DOI] [PubMed] [Google Scholar]
- Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 2006; 49: 170–182. [DOI] [PubMed] [Google Scholar]
- Kwak EK, Kim JW, Kang KS, Lee YH, Hua QH, Park TI et al. The role of inducible nitric oxide synthase following spinal cord injury in rat. J Korean Med Sci 2005; 20: 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara S, Yone K, Setoguchi T, Yamaura I, Arishima Y, Yoshino S et al. Changes in nitric oxide and expression of nitric oxide synthase in spinal cord after acute traumatic injury in rats. J Neurotrauma 2002; 19: 1467–1474. [DOI] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K et al. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res 2000; 85: 114–122. [DOI] [PubMed] [Google Scholar]
- Miscusi M, Ebner F, Ceccariglia S, Menegazzi M, Mariotto S, Berra L et al. Early nuclear factor-kappaB activation and inducible nitric oxide synthase expression in injured spinal cord neurons correlating with a diffuse reduction of constitutive nitric oxide synthase activity. J Neurosurg Spine 2006; 4: 485–493. [DOI] [PubMed] [Google Scholar]
- Osuka K, Watanabe Y, Takagi T, Usuda N, Atsuzawa K, Yoshida J et al. Activation of endothelial nitric oxide synthase following spinal cord injury in mice. Neurosci Lett 2008; 436: 265–268. [DOI] [PubMed] [Google Scholar]
- Lin CL, Su YF, Dumont AS, Shih HC, Lieu AS, Howng SL et al. The effect of an adenosine A1 receptor agonist in the treatment of experimental subarachnoid hemorrhage-induced cerebrovasospasm. Acta Neurochir (Wien) 2006; 148: 873–879 (discussion 879). [DOI] [PubMed] [Google Scholar]
- Yang JY, Kim HS, Lee JK. Changes in nitric oxide synthase expression in young and adult rats after spinal cord injury. Spinal Cord 2007; 45: 731–738. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz A, Ibarra A, Perez-Severiano F, Guizar-Sahagun G, Grijalva I, Rios C. Constitutive and inducible nitric oxide synthase activities after spinal cord contusion in rats. Neurosci Lett 2002; 319: 129–132. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res 2004; 995: 76–83. [DOI] [PubMed] [Google Scholar]