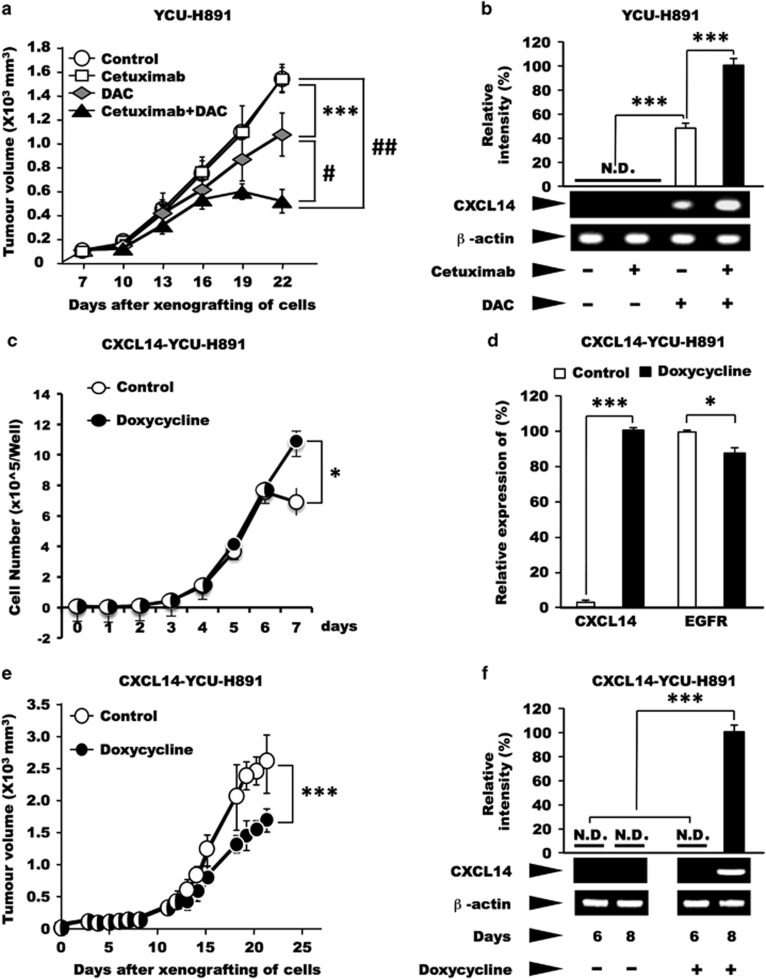
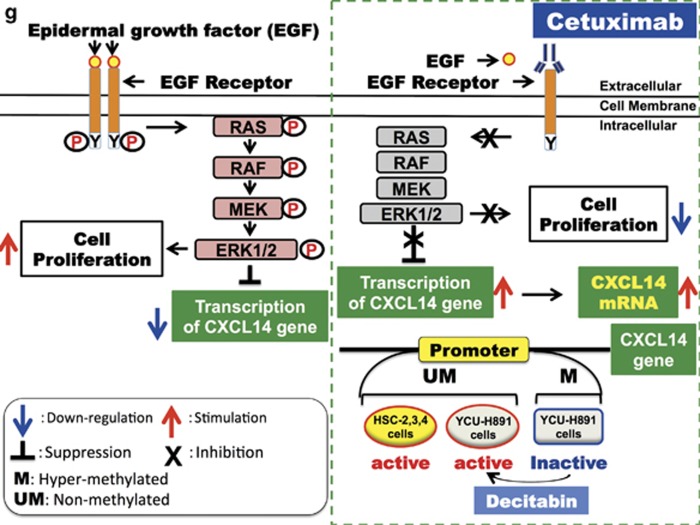
Figure 2.
Sequence analysis of signalling molecules downstream of the epidermal growth factor receptor (EGFR) and the effects of inhibitors of PI3K, MEK and ERK MAPK kinases on CXCL14 expression. (a) After DNA extraction, the nucleotide sequences commonly mutated in cetuximab-resistant colon cancer patients were analysed with a Gene JET Genomic DNA Purification kit (Thermo Fisher Scientific). After PCR amplification of the target sequences, the products were purified with the QIAquick Gel Extraction kit (Qiagen, Tokyo, Japan). We used the following primers for the PCR analyses: 5′-ACA CGT CTG CAG TCA ACT GG-3′ (forward) and 5′-GTC CTG CAC CAG TAA TAT GC-3′ (reverse; PCR product size, 338 bp) for KRAS codons 12 and 13; 5′-CTT TTC AGG TGC TTA GTG TC-3′ (forward) and 5′-AGC AAG TTA CTC CAC TGC TC-3′ (reverse; PCR product size, 538 bp) for KRAS codon 61; 5′-TTT TAT GAC AAA AGT TGT GGA CAG G-3′ (forward) and 5′-CCA AAG CCA AAA GCA GTA CC-3′ (reverse; PCR product size, 431 bp) for KRAS codon 146; 5′-GAA AGC ATC TCA CCT CAT CC-3′ (forward) and 5′-TAA TGG CTG TGG ATC ACA CC-3′ (reverse; PCR product size, 823 bp) for BRAF codon 600; 5′-GCT TTT TCT GTA AAT CAT CTG TGA ATC C-3′ (forward) and 5′-TGC AGA AAT GCA CTG CAA CTG G-3′ (reverse; PCR product size, 672 bp) for PIK3CA codons 542 and 545; and 5′-GCT TTG TCT ACG AAA GCC TC-3′ (forward) and 5′-GCT ATC AAA CCC TGT TTG CG-3′ (reverse; PCR product size, 560 bp) for PIK3CA codon 1047. The PCR cycling conditions were as follows: denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and elongation at 72 °C for 30 s. We used each of the forward and reverse primers to perform the sequencing reactions. We performed the analysis using a dye terminator cycle sequencing kit (Beckman Coulter, Tokyo, Japan) and a CEQ2000 sequencer (Beckman Coulter). The results of direct nucleotide sequencing of the following nucleotides are presented: KRAS codons 12, 13, 61 and 146; BRAF codon 600; and PIK3CA codons 542, 545 and 1047. These regions were not mutated in the HNSCC HSC-3 or YCU-H891 cells. (b–f) To investigate the effects of inhibitors of signalling molecules downstream of the EGFR on CXCL14 expression, we cultured HSC-3 and YCU-H891 cells to the pre-confluent state as described in the Figure 1d legend, and treated with the PI3K inhibitor AS605240 (Echelon Biosciences, Salt Lake City, UT, USA, 10 μM; b) MEK inhibitor PD98059 (Merck Millipore, Darmstadt, Germany, 50 μM; c), MEK1/2 inhibitor U0126 (Merck Millipore, 10 μM; d) or ERK1/2 inhibitor FR180204 (Merck Millipore, 10 μM, e). Total RNA was extracted with TRIzol after 24 h of culture, and the expression levels of CXCL14 and β-actin were determined by RT–PCR. For the HSC-3 cells (b–e) and YCU-H891 cells (f), β-actin cDNA was used as an internal standard and for normalisation. For quantitative comparison of the expression levels of CXCL14 mRNA, qPCR and/or densitometry after agarose gel electrophoresis was performed. The values are expressed as the means±s.d. (n=3). ***P<0.001 (Student's t-test). Experiments were performed in triplicate, and the values were normalized to β-actin.