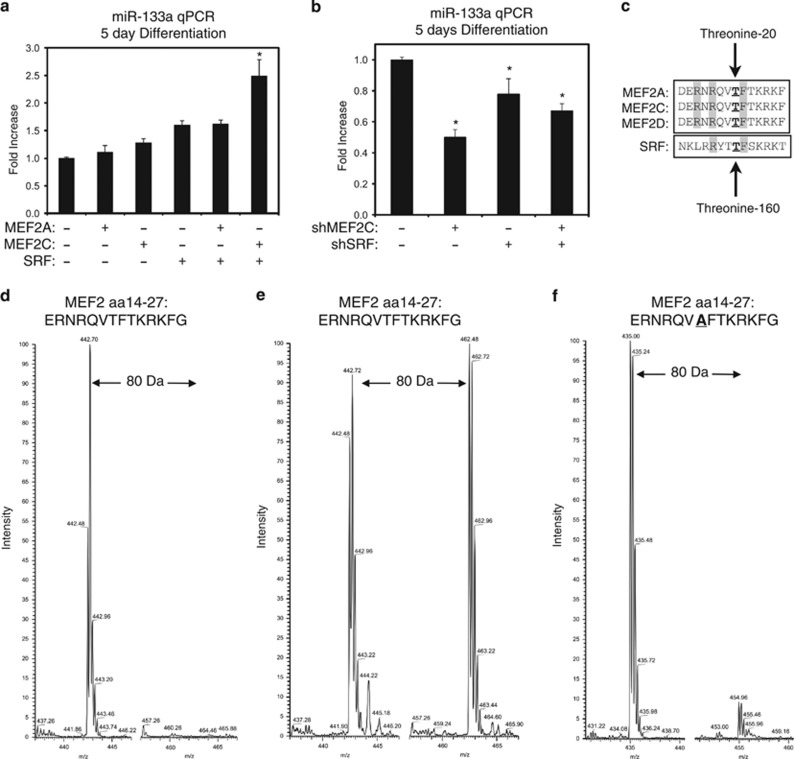
Figure 1.
PKCδ inhibits the cooperation between MEF2 and SRF by direct phosphorylation. (a) C2C12 myoblasts were transfected with MEF2A, MEF2C, or SRF, as indicated. Following recovery, cells were differentiated in low serum media for 5 days and harvested for RNA. Quantitative PCR assays were performed using the ΔΔCT method, where RNU6 was used as an internal control. (b) C2C12 cells were transfected with shRNAs targeting MEF2C (shMEF2C) or SRF (shSRF), as indicated. Following 5 days of differentiation, cells were harvested and assayed as described above. (c) Schematic demonstrating the conservation surrounding the MEF2 threonine-20 and SRF threonine-160 phosphorylation motif. (d–f) SIM scans of the wild-type peptide (d and e) spanning the MADS-box motif of MEF2A. The unphosphorylated peptide (left) has 442 m/z, while the putative phosphorylation (right in b) showing an increased m/z of 20 that corresponds to PO3 (M=80.00 Da). (f) A SIM scan of a mutated peptide where threonine-20 is replaced with alanine is shown. On the right, phosphorylation of this mutate peptide is negligible at the predicted m/z that corresponds to the addition of a PO3 (M=80.00 Da). Both peptides are quadruply charged (z=4+). Data are represented as mean±S.E.M. *P<0.05 compared with control