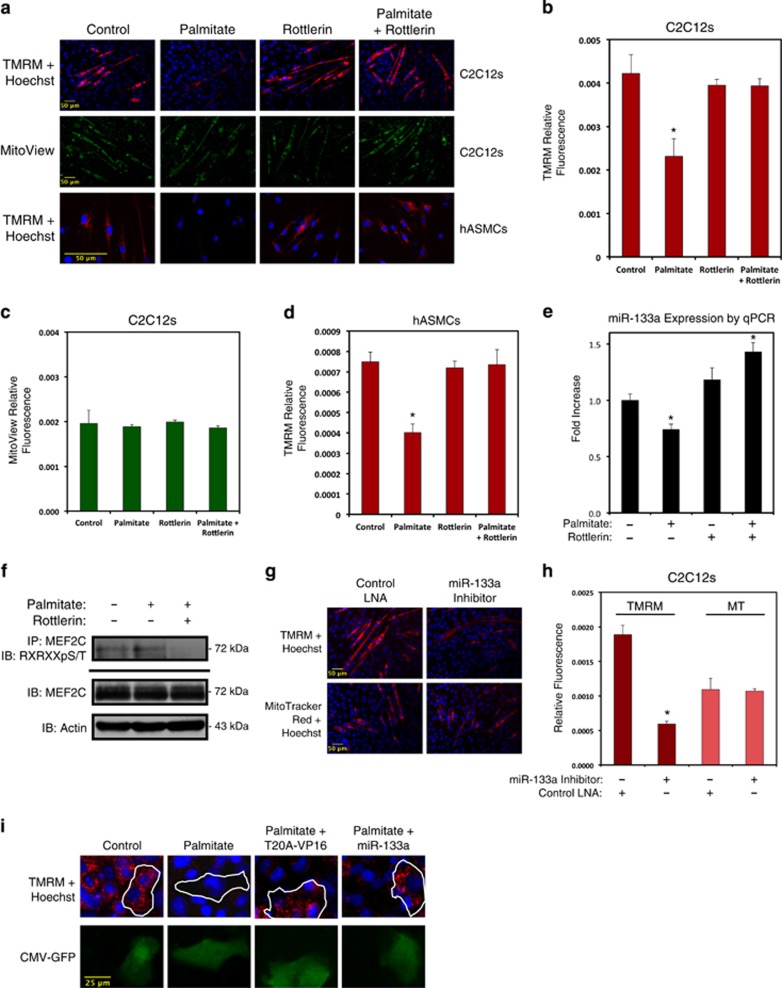
Figure 4.
Palmitate-induced PKCδ activation regulates mitochondrial membrane potential through miR-133a. (a) C2C12 myotubes were differentiated for 5 days (above), or hASMCs were differentiated for 2 days (below), and treated with 200 μM palmitate conjugated to 2% albumin in low glucose media with or without rottlerin (5 μM) overnight. Control cells were treated with 2% albumin alone. Cells were stained with TMRM, Hoechst, and MitoView Green, as indicated, and imaged by standard fluorescence techniques (20 × for C2C12s; 40 × for hASMCs). (b–d) Fluorescent intensities from myotubes in (a) were quantified using ImageJ software (NIH). (e) hASMCs were treated as described in (a), harvested for RNA and subjected to qPCR analysis for miR-133a. (f) Differentiated C2C12 myotubes were treated as in (a). Protein extracts were immunoprecipitated (IP) with an MEF2C antibody, and probed using an antibody that recognizes phospho-serines/threonines with arginines at the –5 and –3 positions (RXRXXpS/T), and immunoblotted (IB), as indicated. (g) C2C12 cells were transfected with a miR-133a inhibitor oligonucleotide or a control oligonucleotide. Cells were differentiated for 5 days and imaged at 20 × with TMRM, Hoechst, or MitoTracker Red CMXRos, as indicated. (h) Quantification of myotube fluorescence in (g). (i) H9c2 myoblasts were transfected with MEF2-T20A-VP16 (T20A-VP16) or miR-133a. Following 2 days of differentiation, cells were treated with 200 μM palmitate conjugated to 2% albumin or 2% albumin alone as a control. CMV-GFP was included to visualize transfected cells. Data are represented as mean±S.E.M. *P<0.05 compared with control