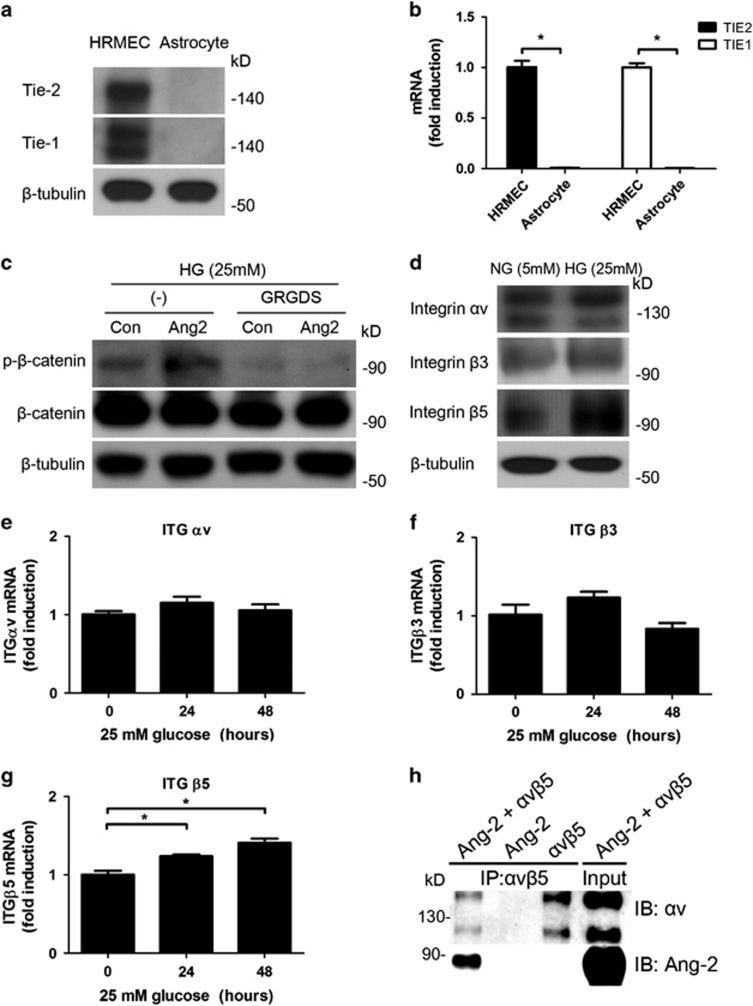
Figure 5.
High glucose increases integrin αvβ5 as an Ang2 receptor in astrocyte. (a) Western blot for Tie-2 and Tie-1 expression were performed on lysates obtained from HRMECs and astrocytes. (b) TIE2 and TIE1 mRNA transcriptions were assessed by quantitative RT-PCR. TIE2 and TIE1 mRNA levels were normalized to β-actin mRNA and reported as fold induction compared to HRMECs. *P<0.001 by Student's t-test. (c) Western blot analysis for phosphor-β-catenin (Thr41/Ser45) and β-catenin were performed on lysates obtained from astrocytes treated with Ang2 (300 ng/ml) or GRGDS (0.5 mg/ml) for 30 min after 48 h of 25 mM high-glucose (HG) incubation. (d) Western blot analysis for integrin αv, β3, and β5 were performed on lysates obtained from astrocytes under HG for 48 h. β-Tubulin was used as a loading control. Data represent three independent experiments. ITGαv (e), ITGβ3 (f), and ITGβ5 (g) mRNA transcriptions were assessed and normalized to β-actin mRNA by quantitative RT-PCR. All mRNA levels were reported as fold induction compared to normal glucose (NG, 5 mM). *P<0.05 by Student's t-test. (h) After incubation of Ang2 (500 ng), integrin αvβ5 (500 ng) and integrin αvβ5 antibody (2 μg), the immune complexes were co-immunoprecipitated to show direct binding of Ang2 to integrin αvβ5. Then, they were analyzed for Ang2 and integrin αv. Same amounts of recombinant Ang2 and integrin α3β1 were used as an input. Con, control