Abstract
Understanding the multiplicity of ways in which sex can alter the brain is essential to crafting policies and treatments that are beneficial for all human beings. This is particularly true for the field of neuropsychopharmacology, as many neuropsychiatric disorders exhibit gender bias in the frequency, severity, or response to treatment. The goal of this circumspective is to provide two views on the current state of the art of the relations between sex and the brain, relations that are studied almost exclusively by comparing females and males on specific end points, from gene expression to behavior. We start by suggesting a framework for defining what is being measured and what it means. We suggest that ‘sex differences' can be classified on four dimensions: (1) persistent vs transient across the lifespan; (2) context independent vs dependent; (3) dimorphic vs continuous; and (4) a direct vs an indirect consequence of sex. To accurately classify a sex difference along these dimensions, one may need to compare females and males under varied conditions. We next discuss current data on the mechanisms of sexual differentiation of the brain and on sex differences in the brain to conclude that the brain of each male and female is a mosaic of relative masculinization, feminization, and sameness, which theoretically could produce an infinite variety of individuals. We also raise the possibility that sex differences in the brain are canalized, which may act to both enhance and restrain variation between males and females. We end by discussing ways to consider sex when studying neuropsychiatric disorders.
Preamble
The question of whether the distinction between males and females extends into the brain is as old as the first scientific discourse and a hot new topic. The existence of differences between brains of females and brains of males is not debated. It is the origins, meaning, and magnitude of sex differences that are often points of discord between scientists and a source of hyperbole and exaggeration in the media. Understanding the multiplicity of ways in which sex can alter a physiological or behavioral response is essential to crafting appropriate policies and treatments that are beneficial and protective of all human beings. This is particularly true for the field of neuropsychopharmacology, which seeks to understand and develop therapeutics for disorders that exhibit gender bias in the frequency, severity, or response to treatment. The recent policy shifts of major granting institutions in the US, Canada, and the European Union calling for increased attention to sex as a biological variable further raises the gain for appropriate measurement and analyses of brain and behavior in human and non-human females and males.
The goal of this circumspective is to provide two views on the current state of the art of the relations between sex and the brain. Our views are at times complementary and overlapping, and at other times quite distinct or even opposing. Abiding by the data is the principle that guides us both. The existence and meaning of sex differences in the brain are questions of such broad ranging significance and impact that is essential for it be assessed with utmost rigor. It is a topic worth of attention.
A framework for interpreting sex differences
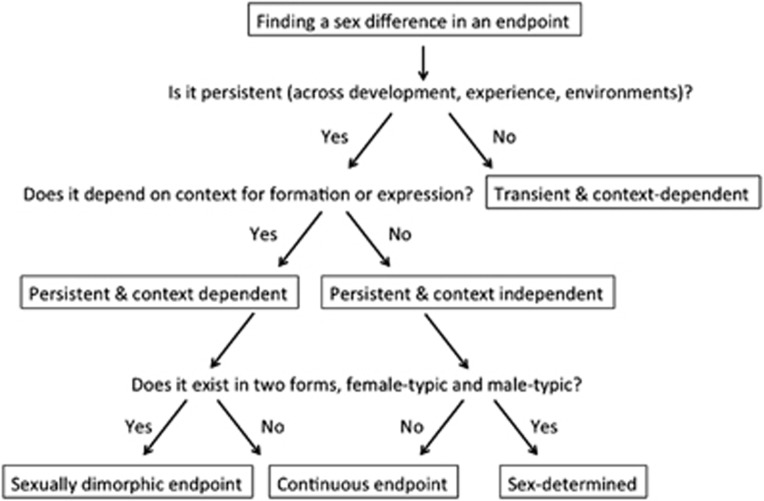
Appropriate interpretation of the relations between sex and the brain requires a framework for defining what is in fact being measured and what it means. To date, the relations between sex and the brain are studied almost exclusively by comparing females and males on specific end points, from gene expression to behavior, and reporting differences between the two groups. We suggest that such ‘sex differences' can be classified on four dimensions: (1) persistent vs transient across the lifespan; (2) context independent vs dependent; (3) dimorphic vs continuous; and (4) a direct vs an indirect consequence of sex (Figure 1).
Figure 1.
A framework for interpreting sex differences. The effects of sex are typically studied by comparing a group of females with a group of males on specific end points. The diagram charts a series of questions needed for characterizing the sex difference observed in regards to the dimensions of persistent vs transient, context dependent vs context independent, and whether the end point is sexually dimorphic or continuous.
Dimensions 1 and 2: Persistent vs Transient, Context-Independent vs -Dependent Sex Differences
Building on an analogy to the sexual differentiation of the genitalia, the classic view of the sexual differentiation of the brain posits that sex differences emerge already in utero and persist largely unchanged throughout life. This is true for some neuroanatomical end points and the physiology and/or behaviors they control, particularly in the realm of reproduction (Simerly, 2002; McCarthy and Rissman, 2014). However, in many instances, both neural and behavioral sex differences change throughout the life span. Such changes may take place at different developmental stages (eg, the sex difference in the size of the bed nucleus of the stria terminalis appears in humans only at adulthood (Chung et al, 2002)), or following a change in specific internal or external factors at a given developmental stage (eg, the density of dendritic spines in the rat hippocampus fluctuates across the female estrous cycle, and is also modulated by stress in both females and males (Woolley et al, 1990; Shors et al, 2001)). Thus, a sex difference might be apparent at one age and not another, or evident under stressful conditions but not in relaxed conditions. Such sex differences are therefore both transient and context dependent, with that context being age, hormonal milieu, experience, and environment. Some persistent sex differences, ie, sex differences that are established at a specific stage of development and endure throughout life, may also be context dependent, either at the time of their formation or of their expression. An example of the former would be the dependence of the size and direction of some sex differences on the circumstances at the critical time window in which they were established (eg, see Richardson et al, 2006; Rothstein et al, 2008). An example of a sex difference that is apparent only under certain circumstances is the sex-specific neuroanatomical underpinnings of some parental behaviors, which are established neonatally, but the behavior is not expressed before the appropriate activational hormonal milieu is established following mating/pregnancy (Dulac et al, 2014; Scott et al, 2015). The same is true for mating behavior in that animals must be reproductively mature to copulate but the neural underpinnings are established perinatally and consist at least in part of sex differences in synaptic patterning in key brain areas (McCarthy et al, 2015).
To determine whether a sex difference is persistent or transient, context dependent or context independent, it is necessary to compare females and males under varying pre-natal and post-natal conditions established by manipulating environmental conditions (eg, group vs individual housing, different levels of stress), as well as at various ages.
Dimension 3: Dimorphic vs Continuous End Points
For an end point to be truly sexually dimorphic, it needs to exist in two forms, one more prevalent in one sex vs the other. Sex behavior in rodents is an example, as there is a male-typic and a female-typic behavior, even if males and females occasionally exhibit the opposite sex behavior. Another example is the size of the sexually dimorphic nucleus of the preoptic area in rodents that is established within the first week of life and remains larger in males into full adulthood (Gorski et al, 1980). But for the most part, sexual dimorphisms are infrequent and instead the sexes differ on average along a continuum of a particular end point, be it behavioral, neuroanatomical, or physiological. Sometimes there is a great deal of overlap, with many males showing similar responses to many females. Referring to such differences as a sex dimorphism is inappropriate and misleading (McCarthy and Konkle, 2005; Joel, 2012).
A sexually dimorphic end point that is not reversed by context and is persistent throughout life would be termed sex determined, because its form is determined by the sex category of the subject. The example above of the neural circuitry controlling some aspects of parental and mating behavior can be considered sex determined, even though there remains some degree of plasticity as there would with any neural circuit.
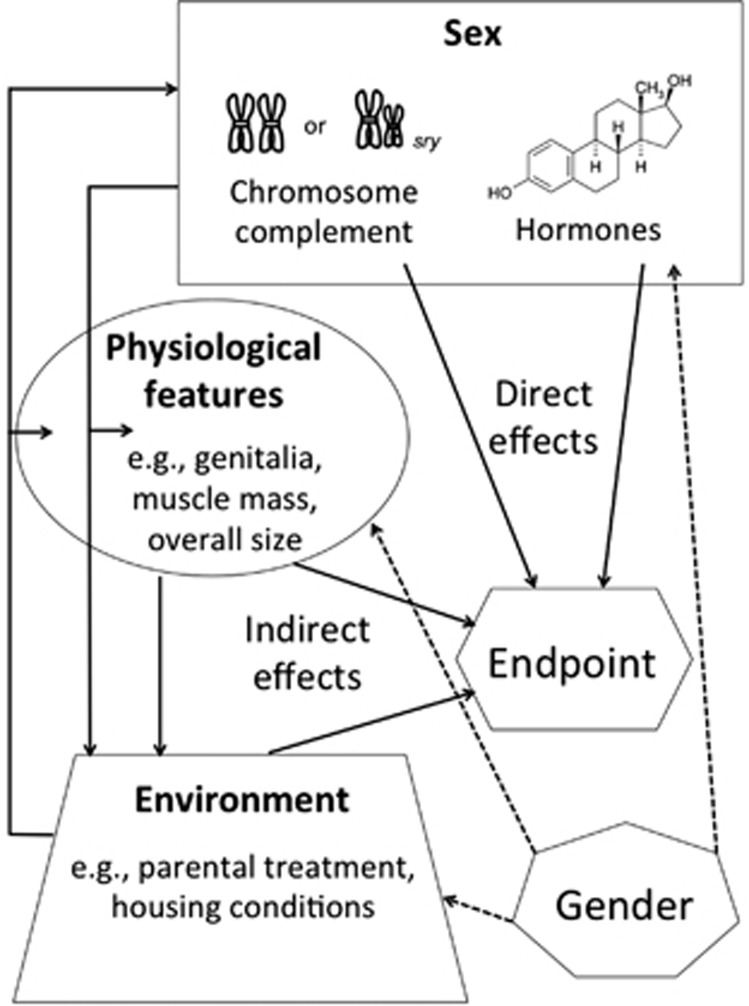
Dimension 4: Direct vs Indirect Origins of Sex Differences
There are only two sources of biological influences of sex on the mammalian brain: (1) XX vs XY and (2) gonadal hormones (Figure 2). But there are a myriad of additional factors that can cause or influence differences between females and males. These include differences in the genitalia, overall size, muscle mass, and sexually selected traits such as plumage color, antlers, canine size, body hair distribution etc. All of these can impact on behavior, as the brain cannot be separated from the influence of the body it inhabits (de Vries and Forger, 2015). These physical attributes also impact how conspecifics interact with any given individual. In humans this is a component of gender, meaning societies' expectations and therefore modulation of behavioral norms for males vs females. In humans and animals, the sex of a newborn impacts the way parents behave toward the infant, thereby modifying brain development (Moore and Morelli, 1979; McHale et al, 1999; Bowers et al, 2013). Thus, the detection of a difference between a group of females and a group of males in an end point may reflect the direct effects of sex chromosomes and/or sex hormones on that end point, or the effect of some variable that is correlated with the sex category. In the latter case, this variable may be a direct biological consequence of sex (eg, plumage color and antlers) or a component of gender (eg, socioeconomic status). As has been noted by several authors, it is impossible to disentangle sex and gender in humans (eg, Rippon et al (2014)). However, as both scientifically and clinically it is important to understand the origins of observed sex differences in brain and behavior, it is important that studies of such differences measure or manipulate relevant internal and external variables and not assume that a sex difference necessarily reflects a direct sex effect (Rippon et al, 2014; Joel and Fausto-Sterling, 2016).
Figure 2.
Direct and indirect effects of sex. The effects of sex on brain and behavior can be direct (ie, hormones and chromosome complement) or indirect, through sex differences in other physiological factors or in environmental factors. The direct effects of sex chromosomes and sex hormones may be synergetic or antagonistic. The indirect effects may exaggerate the direct effects of hormones or chromosome complement or act independently of other influences of sex to modulate brain and behavior. The physiological and the environmental factors through which sex may indirectly affect brain and behavior may themselves be a direct consequence of sex (eg, muscle mass, antlers, and parental behavior), or, in humans (dashed lines), may also be a consequence of gender (eg, sex roles and socioeconomic status). In addition to influencing physiological and environmental factors, components of gender may also affect components of sex itself (eg, level of testosterone).
In summary, although to date the kingsroad to study sex effects on the brain is to compare males and females on specific end points, the above discussion highlights that the existence of a sex difference in an end point does not necessarily entail that sex directly affects this end point or that this effect is permanent and will be seen under all conditions. Therefore, although many sex differences have been observed at different levels, from gene expression, through morphology, to behavior, suggesting that sex affects the brain, much work is still needed to better characterize these differences—which reflect the direct effects of sex and which reflect only variables that co-vary with sex, which are permanent and which transient, which are context dependent and which are not, and which reflect a shift in the distribution of females relative to males or vice versa and which are a true sex dimorphism. Such characterization is necessary for unraveling the relations between sex and the brain in health and pathology. Moreover, these considerations highlight the need to develop additional experimental methods for studying sex effects.
Is there a male brain and a female brain?
McCarthy
My personal research goal is to discover the basic cellular and molecular mechanisms by which sex differences in the brain are established by hormones early in development. Within this framework, we have identified multiple signal transduction cascades that are induced by estradiol. These include activation of specific kinases, synthesis, and activity modulation of various enzymes, changes in structural and anchoring proteins, induction of transcription factors and epigenetic modifications (McCarthy et al, 2015). The striking thing is not the diversity of mechanisms as much as it is the highly region specific distribution of distinct mechanisms. For instance, a signal transduction cascade mediated by PKA might be essential in one region, whereas IP3 kinase is in another and so on, with no overlap or commonality between them. Others have seen the same thing and even within a region mechanisms will vary yet be mediated by the same steroid. In one particularly striking example, there is a hypothalamic nucleus that is larger in females because of cell death in that region in males. Both dopaminergic and GABAergic neurons die in male early in the development, but the mechanism of cell death is entirely different for each cell type (Krishnan et al, 2009; Waters and Simerly, 2009). A laundry list of all of the distinct cellular mechanisms mediating neuroanatomical and neurophysiological end points would fill pages and remain incomplete, as there is surely more to be discovered. But the larger message in this multitude of mechanisms is the unavoidable conclusion that there cannot be uniform masculinization or feminization of the entire brain. With so many different points of variability, both in allelic or imprinting genetic variants and in sensitivity to internal and external factors, it is inevitable that within the brain of a male there will be some regions that are strongly masculinized, whereasa others will be less so or even skewed toward feminized and vice versa. Thus, the brain of each male and female is a mosaic of relative masculinization, feminization, and sameness. The result is an infinite variety of individuals both within and between the sexes.
Now I am going to appear to contradict myself. A mosaic is not the same thing as a blend. A blend is a smooth continuum of change and in the context of sex differences would suggest that males and females seamlessly vary from one end to the other. If true, this should be evident in the data. Lets take an imaginary end point that is on average different in males and females. If the distribution of values for the end point in males and females lies along a continuum, then quantification of a small number of subjects would be predicted to detect a small sex difference in the mean and a high degree of variability. If, however, the imaginary end point is even weakly bimodal in its distribution based on sex, a sampling of a small number of subjects would result in a large magnitude average difference and small variability. This would only increase the more sharply bimodal the distribution. We have found this to be the case for a large number of sexually differentiated end points in the developing rat brain, ranging from density of dendritic spines, to morphology of astroglia and microglia to rates of cell genesis (see for review McCarthy (2016)). Moreover, we also find that when we induce masculinization in neonatal females by means of hormone injections, we never produce a ‘super-male', meaning the end point never exceeds that achieved normally in males. So either all masculinized end points in males are at a maximum, which seems unlikely, or some factor is acting as a governor to prevent further masculinization of the end point. Likewise, we rarely see end points in females that become more extreme in response to some manipulation. So either all feminized end points are also at a maximum, or some factor is acting as a governor to prevent further feminization or defeminization in females. It is possible that this is exaggerated by or even an outright artifact of the constrained conditions under which we rear laboratory animals. But it may also be biological. This combination of an apparent bimodal distribution and an intrinsic limitation on the ranges is suggestive of the process of canalization. Canalization was first coined as a term by Waddington to connote the process of differentiation, whereby once a particular path is chosen others are excluded (Waddington, 1959). More recently, canalization is evoked to explain the robustness of species in the face of continuous onslaught by agents of change (Rohner et al, 2013). Robustness refers here to the maintenance of a narrow phenotype. Organisms are constantly challenged by changes in the internal and external environment and spontaneous genetic mutations. Robustness in the face of these challenges prevents individuals from drifting out of the range of phenotypic variation for that species. A number of different agents mediate robustness. The two most well characterized are chaperone proteins, which maintain proper protein folding and trafficking, and microRNAs which regulate transcription and often impose a threshold for protein synthesis (Ruden et al, 2003; Posadas and Carthew, 2014). I propose that sex differences in the brain are also subject to canalization to assure that males and females are robustly different on multiple end points, but to also assure not too much. The sexes need to stay within range of each other. This could be simply for the sake of optimal physiological and behavioral functioning, which provides the basis for the boundaries. It could also be to avoid a significant enough divergence in male and female performance that they were no longer reproductively compatible. Whether or not sex differences in the brain are canalized is an empirically testable question and the answer awaits future research.
Joel
In recent years, I have been attempting to answer whether sex differences in the brain ‘add up' to create two types of brains, ‘male' and ‘female'. For this to be true, the effects of sex should be dimorphic, ie, result in the formation of distinct ‘male' and ‘female' types, and internally consistent, ie, that all elements of a single brain are either ‘male' or ‘female' (Joel, 2011, 2012; Joel and Fausto-Sterling, 2016). As McCarthy reviewed above, the complexity and independence of masculinization and feminization of different brain tissues predict poor internal consistency in the degree of ‘maleness–femaleness' of different features within a single brain (Joel, 2011, 2012). Moreover, evidence that the effects of sex may be opposite under different environmental conditions and that these sex-by-environment interactions may be different for different brain features suggests that brains are comprised of a ‘mosaic' of features, some more common in males and others more common in females. We have recently found that in the human brain—having great variability in the degree of ‘maleness–femaleness' of different features within a single brain was much more common than having internal consistency in the degree of ‘maleness–femaleness' of different features. This was true regardless of sample, age, imaging method (T1-weighted or diffusion tensor), or type of analysis (volume based, surface based, or connectivity). In all cases, most brains were comprised of unique mosaics of features, some more common in males compared with females, others more common in females compared with males, and others common in both females and males (Joel et al, 2015). It thus seems that rather than consisting of two distinct populations of brains, ie, ‘male brains' and ‘female brains', the population of human brains consists of many ‘types' of brains that are similarly common in females and males, and some rare ‘types' of brains, which are more likely in one sex over the other (eg, although brains in which all features are consistently towards the ‘male-end' of the ‘maleness–femaleness' continuum are rare, they are more common in males compared with females (Joel and Fausto-Sterling, 2016)).
The question of whether brains of females and males belong to two distinct populations has implications for studies of the relations between sex and the brain as well as for studies of brain structure and function in general (Joel and Fausto-Sterling, 2016). This is because if brains belong to two distinct populations, then the sex category should be included as a variable in every analysis of brain structure and function to control for sex-related variability. On the other extreme, if brains belong to a single population, then including a binary sex category as a variable is not only unnecessary but would most likely result in the discovery of false sex differences, ie, differences that reflect a chance difference between the group of females and the group of males included in the study (Joel, 2011; Joel and Fausto-Sterling, 2016). Further studies are needed to unravel how best to characterize the population of human brains—do they belong to a single population, to several distinct populations (ie, distinct brain types), or to two distinct types? And a similar analysis should be done for brains of other animals.
Last, our work has revealed that sex differences in the brain do not ‘add up' structurally—ie, the degree of ‘maleness–femaleness' of one brain feature showing a sex difference does not necessarily match the degree of ‘maleness–femaleness' of another brain feature showing a sex difference (Joel et al, 2015). Others have already pointed out that sex differences in the brain also do not ‘add up' functionally—ie, a sex difference may be functionally ‘compensating' for another sex difference, rather than adding to it to make the two sex categories more distinct (de Vries, 2004).
Future directions: how should we consider sex when studying neuropsychiatric disorders?
McCarthy
The US National Institutes of Health (NIH) mandated that women be incorporated into clinical trials almost 25 years ago. As a result, the representation of women in NIH funded research trials increased markedly, but our understanding of differences in the health of men vs women did not. Gender bias in neuropsychiatric disorders is pervasive and robust. Males suffer disproportionately from disorders with origins in development, whereas females are over-represented in those with adult onset (Bao and Swaab, 2011). Yet the enormous tractability of comparing neural and behavioral processes in males vs females in the preclinical realm has been largely ignored. The reasons for this are unclear but likely have origins in the assumption that sex differences in the brain are limited to the control of reproduction and that neural substrates mediating more complex cognitive and emotional response profiles would not differ. The discovery of robust neural plasticity in the adult hippocampus mediated by the gonadal hormone, estradiol, up ended this assumption, and opened the door to the possibility of sex differences in the realm of cognition, emotionality, sociability, and stress responding. The initial studies from the McEwen lab reported surprisingly high degrees of neural plasticity across the female estrus cycle (Woolley and McEwen, 1992). In retrospect, this may have further contributed to the exclusion of females from preclinical studies as researchers sought to avoid the additional variability introduced by including both sexes. Thus, despite the persistent reports of profound sex differences in neuroanatomical, neurophysiological, and behavioral end points relevant to neuropsychiatric disorders, the representation of females was driven even lower. An analysis of research published in 2009 in the neurosciences and pharmacology (which relied heavily on psychopharmacology journals) found 50–60% used only male animals, whereas an alarming 10–25% did not note the sex of the animal they were using. In the field of physiology, 12.5% of studies included both sexes and 30% of those looked for a difference, meaning only 3.75% of studies incorporated sex as a biological variable. Estimates are slightly better but comparable for neuroscience and psychopharmacology (Beery and Zucker, 2010).
The question now is what is the best way to rectify this gap going forward. The challenges are both daunting and more easily addressed than one would assume. First is to dispense with the notion that all studies must include females in equal numbers and in all stages of the estrus cycle, essentially quadrupling the n. Retrospective analyses of both behavioral and genomic studies reveal that the variability introduced by the female estrus cycle is minimal and at times surpassed by the variability introduced due to housing conditions (ie, grouped vs isolated; Prendergast et al, 2014). Second is that everything does not need to be repeated in females. Instead, as studies move forward, there should be inclusion of some female subjects and a post hoc analysis that includes sex as a biological variable. A number of recent publications offer guidance on sample size and power analyses etc. But the important point is that studies do not need to be powered to detect a sex difference, they need to be powered to detect effects of the manipulation under study and to incorporate sex as a variable (McCarthy, 2015). But there are some serious challenges. Behavioral readouts for assessing depressive or anxious behavior in animals are not always applicable to both sexes. The social-defeat model in mice, which has provided excellent traction for understanding the neural basis of depression (Golden et al, 2011), cannot be used in females. Freezing behavior measured in conditioned fear tests is also not appropriate to females (Gruene et al, 2015). Sex differences in the Morris water maze are more a by-product of the stress than actual spatial learning (Perrot-Sinal, 1996), and the list goes on. This does not mean that these behavioral assays are not valuable, but it does demand that we constrain our conclusions about the generalizability of findings and that when necessary, we develop new tests to assess the effects in females. Increased awareness of the importance and the heuristic value of including females in preclinical research will be to the benefit of all neuropsychiatric research.
Joel
I think answering this question is the major challenge of scientists studying the relations between sex and the brain as well as between sex and other systems. I also think we need to study how to study sex, because current data do not suffice to answer this question. To date, we do not assess sex, ie, genes and hormones, but rather use the sex categories (female and male) as a proxy of sex (Maney, 2016). This is however a very poor proxy because it does not capture the interactions between the different components of sex (eg, Arnold et al, 2013) nor their dynamic and reactive aspects (eg, the wide fluctuations in the level of the three main gonadal hormones, estradiol, progesterone, and testosterone, within individuals of both sex categories as a function of multiple factors, including environmental (eg, time of day) and social (eg, status; van Anders et al, 2014)). Future studies would hopefully advance our understanding of these aspects of sex, and lead to the development of methods for incorporating measures of sex into studies of brain and behavior. Only by correlating direct measures of sex (eg, expression of genes and level of hormones) with measures of brain and behavior, and by assessing the effects of manipulations of the different components of sex on brain and behavior, can we appreciate sex effects on the brain.
Until such methods are routinely used, it is important that we are always aware of the limitations of the current practice of comparing females and males as a means to unravel sex effects, and avoid interpreting every sex difference as revealing direct, context-independent and persistent sex effects.
I suggest three principles that should govern studies of brain and behavior. First, all studies should include equal numbers of females and males as subjects, to represent a species' variability, regardless of whether sex effects are of interest or not and whether there is evidence that males and females are similar or different in the end point to be measured. Second, as explained above, researchers should consider whether sex category should be included as a variable in the analysis of the results, on the basis of existing knowledge regarding the relations between sex and the end point to be measured (Joel and Fausto-Sterling, 2016). As McCarthy points out above and I have stressed previously (Joel and Fausto-Sterling, 2016), even if sex category is used as a variable, studies which do not aim to assess sex effects (eg, studies of the neural or behavioural effects of a pharmacological manipulation) should not be powered to detect differences between females and males, but rather to detect differences between the different experimental conditions (eg, vehicle-treated vs drug-treated groups). In such studies, however, it is important to check whether the effects of the manipulation are different in females and in males, ie, to look for interactions between sex category and the manipulation (this should be done by looking at the actual data not just the statistics, which at this point may not be powered enough to detect a significant interaction). Only when the data suggest a sex category-by-manipulation interaction, doubling the number of animals is required, to allow the assessment of the effects of the manipulation in females and in males separately. Third, if a difference between females and males is found and is large enough to encourage further investigation, revealing the type of relations between sex and the phenomena under study requires a comparison between males and females under several pre- and post-natal conditions. Although we often implicitly assume that a sex difference reflects a direct effect of sex, which is context independent and persistent, very few sex differences, if at all, have been demonstrated under varied enough conditions to support such an assumption. Testing under multiple conditions is necessary if one is to conclude that a certain end point is persistent, context independent, and sexually dimorphic. It is even more difficult to conclude that a difference between females and males is a direct effect of sex and not of a biological or environmental variable that correlates with sex. In animals, such a conclusion requires that studies either control for physiological variables (eg, weight) and avoid environmental variables (eg, housing conditions) that show sex differences, or systematically manipulate these factors. In humans it is critical to also assess psychological and social variables that correlate with sex category and that may be relevant to the phenomenon under study (Joel and Fausto-Sterling, 2016).
Summary
McCarthy
The field of neuroscience is no longer in its infancy, but it is certainly not mature. We are still largely in a discovery phase, with much of the science directed towards understanding how the brain works at the most basic level under normal conditions. Review articles and textbooks are filling with sweeping statements of fact about the fundamentals, but many of these have not been confirmed to be equally true for males and females. Recent studies have revealed that we can take nothing for granted. Sex differences occur at the molecular, cellular, physiological, and behavioral level, and are pervasive across the brain, lifespan, and context. But does this mean that 40 years of progress needs to be repeated? Few would agree and so the challenge is to strike the right balance between inclusion of both sexes and unimpeded progress. As we have discussed at length throughout this circumspective, end points influenced by sex may be persistent, transient, context dependent, or serve more to bring the sexes together than drive them apart. But this does not negate the importance of a sex difference at any given time for any given end point and this possibility should be considered. To do this, in my opinion, one needs only to include females in the group of subjects being examined. It does not need to be half, but it should not be 10% either. When data analyses are conducted, multivariate statistics that address sex as a biological variable should reveal if this is an important contributor to the response. The study does not need to be powered to detect sex differences, only to detect if sex is a contributing variable (McCarthy, 2015). If it is not, future studies should always include females, because, why not? If sex is an important variable, then the investigator can decide to exploit the heuristic power of comparing males and females, or decide to move on and restrict future conclusions to half the population.
Joel
There is no doubt that the genetic and hormonal components of sex affect many aspects of physiology, including the brain, and that understanding these effects will advance human health. Therefore, there should be no doubt that including female and male subjects in all studies of brain and behavior is necessary. The question is whether and how we should treat sex as a variable in the analysis of the results. The challenge we face is moving away from dominant preconceptions of sex based mainly on an analogy to sex effects on the genitalia, and developing new concepts and methods of investigation that better suit the complex relations between sex and the brain. Efforts should be directed to a better understanding of sex itself, ie, its genetic and hormonal components and their interactions, as well as of the interactions of these components with other genetic, hormonal, developmental, and environmental factors. In parallel, acknowledging the importance of such interactions in the realm of sex and the brain, the current practice of comparing females and males should take context into account, by assessing females and males under multiple conditions, to reveal the type of relations between sex and the brain: persistent or transient, context dependent or independent. That sex effects may be context dependent also means that one cannot easily generalize from a single study to other conditions and other species. Finally, it is important to remember that although comparing females and males is to date the main experimental method for studying sex effects, not every sex difference reflects a genuine sex effect.
Funding and disclosure
The authors declare no conflict of interest.
References
- Arnold AP, Chen X, Link JC, Itoh Y, Reue K (2013). Cell-autonomous sex determination outside of the gonad. Dev Dyn 242: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Swaab DF (2011). Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol 32: 214–226. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I (2010). Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM (2013). Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 33: 3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, de Vries GJ, Swaab DF (2002). Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci 22: 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ (2004). Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145: 1063–1068. [DOI] [PubMed] [Google Scholar]
- de Vries GJ, Forger NG (2015). Sex differences in the brain: a whole body perspective. Biol Sex Differ 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O'Connell LA, Wu Z (2014). Neural control of maternal and paternal behaviors. Science 345: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM (1980). Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol 193: 529–539. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. eLife 4: e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D (2011). Male or Female? Brains are Intersex. Front Integr Neurosci 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D (2012). Genetic-gonadal-genitals sex (3G-sex) and the misconception of brain and gender, or, why 3G-males and 3G-females have intersex brain and intersex gender. Biol Sex Differ 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Fausto-Sterling A (2016). Beyond sex differences: new approaches for thinking about variation in brain structure and function. Philos Trans R Soc Lond B Biol Sci 371: 20150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y et al (2015). Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci USA 112: 15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL (2009). Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA 106: 16692–16697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL (2016). Perils and pitfalls of reporting sex differences. Philos Trans R Soc Lond B Biol Sci 371: 20150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM (2015). Incorporating sex as a variable in preclinical neuropsychiatric research. Schizophr Bull 41: 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM (2016). Multifaceted origins of sex differences in the brain. Philos Trans R Soc Lond B Biol Sci 371: 20150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT (2005). When is a sex difference not a sex difference? Front Neuroendocrinol 26: 85–102. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Rissman EF (2014). Epigenetics of reproduction. In: Plant TM and Zeleznik AJ (eds). Knobil & Neill's Physiology of Reproduction, 4th edn. New York: Academic Press, pp 2439–2501.
- McCarthy MM, Pickett LA, VanRyzin JW, Kight KE (2015). Surprising origins of sex differences in the brain. Horm Behav 76: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale SM, Crouter AC, Tucker CJ (1999). Family context and gender role socialization in middle childhood: comparing girls to boys and sisters to brothers. Child Dev 70: 990–1004. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA (1979). Mother rats interact differently with male and female offspring. J Comp Physiol Psychol 93: 677–684. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS (1996). Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci 110: 1309–1320. [DOI] [PubMed] [Google Scholar]
- Posadas DM, Carthew RW (2014). MicroRNAs and their roles in developmental canalization. Curr Opin Genet Dev 27: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40: 1–5. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL (2006). Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology 147: 2506–2517. [DOI] [PubMed] [Google Scholar]
- Rippon G, Jordan-Young R, Kaiser A, Fine C (2014). Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Front Hum Neurosci 8: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner N, Jarosz DF, Kowalko JE, Yoshizawa M, Jeffery WR, Borowsky RL et al (2013). Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342: 1372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S, Simkins T, Nunez JL (2008). Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience 152: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden DM, Garfinkel MD, Sollars VE, Lu X (2003). Waddington's widget: Hsp90 and the inheritance of acquired characters. Semin Cell Dev Biol 14: 301–310. [DOI] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T (2015). A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525: 519–522. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21: 6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB (2002). Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25: 507–536. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL, Bell SN (2014). Measurement of testosterone in human sexuality research: methodological considerations. Arch Sex Behav 43: 231–250. [DOI] [PubMed] [Google Scholar]
- Waddington CH (1959). Canalization of development and genetic assimilation of acquired characters. Nature 183: 1654–1655. [DOI] [PubMed] [Google Scholar]
- Waters EM, Simerly RB (2009). Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci 29: 9714–9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS (1992). Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12: 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS (1990). Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10: 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]