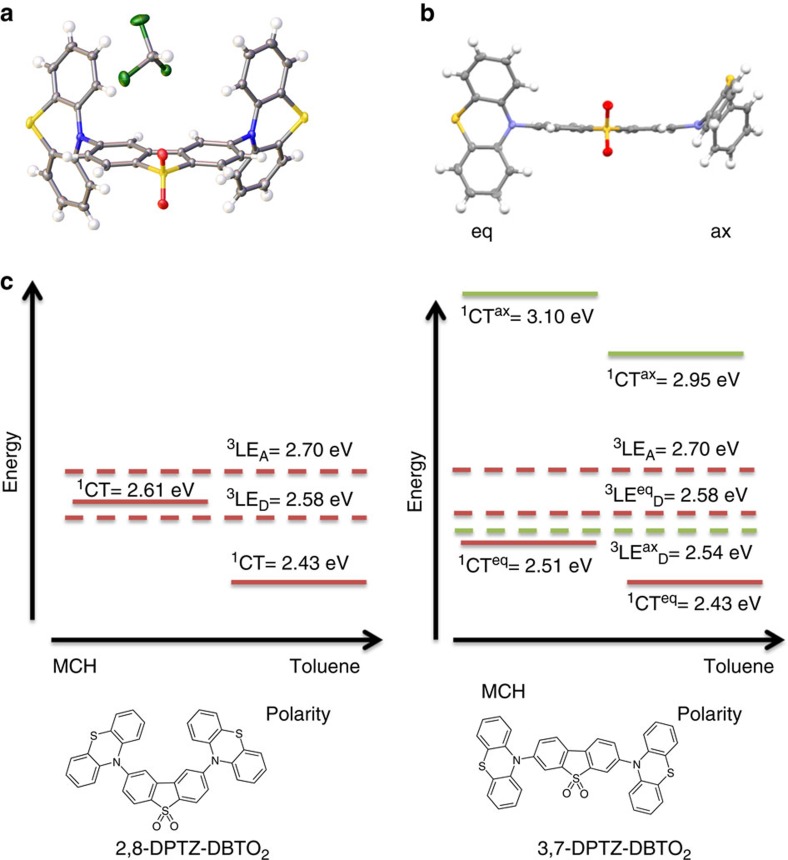
Figure 1. X-ray crystal structures and the energy level arrangement of the two isomers.
X-ray crystal structures of the molecules (a) 2,8-DPTZ-DBTO2 showing an equatorial PTZ conformation and (b) 3,7-DPTZ-DBTO2 having mixed axial and equatorial conformations of PTZ (molecular structure can also be found in Supplementary Fig. 20). (c) Energy positions of the 1CT and 3LE states for the molecules 2,8-DPTZ-DBTO2 (LHS) and 3,7-DPTZ-DBTO2 (RHS). Changing from MCH to toluene for 2,8-DPTZ-DBTO2 moves from type II–III TADF, whereas the equatorial conformer D–A pair (eq)3,7-DPTZ-DBTO2 is type III in MCH and remains so in toluene (see Etherington et al.7 for notation). Vibronic coupling occurs between the CT manifold and the local triplet excitons (see Supplementary Fig. 12). For the axial (ax) conformer pair the gap is >0.5 eV and vibronic coupling is suppressed. These values have been extracted from the onset of the 1CT emission (Fig. 2b and Supplementary Fig. 6b) and the phosphorescence of the 3LE state measured in zeonex (Fig. 6a).