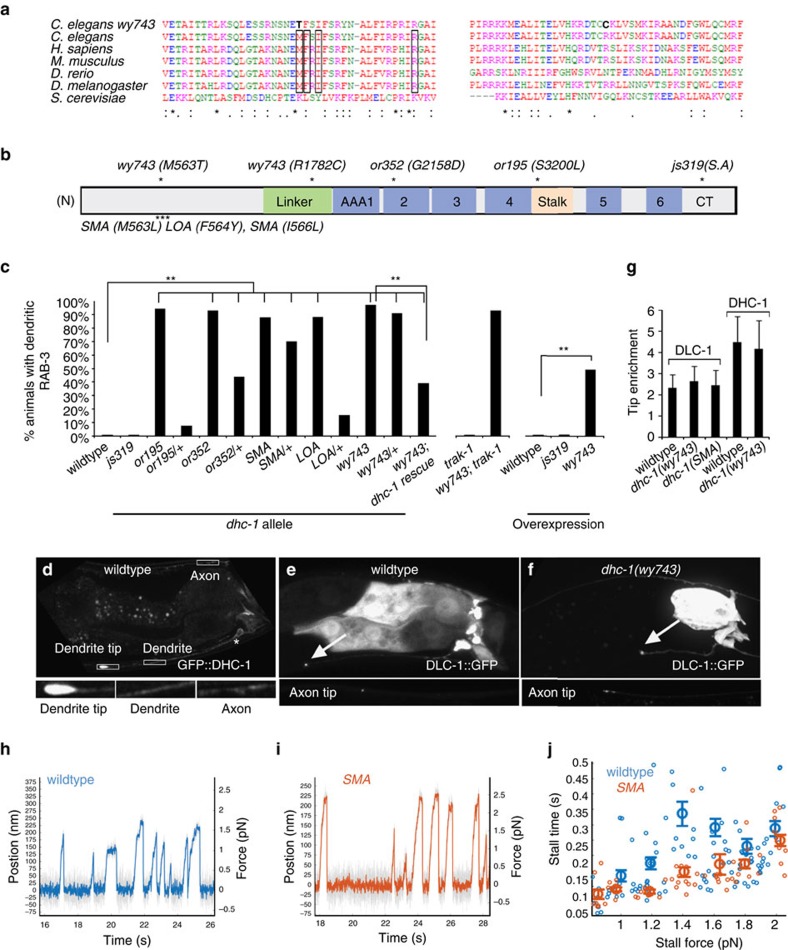
Figure 2. wy743 is an allele of dhc-1.
(a) Alignment of dynein heavy chain sequences encoding the N terminal (left) and linker domains (right), shows their high conservation. The mutated residues in wy743 are highlighted. Boxes mark mutations that are associated with neuronal dysfunction in mice and humans. (b) Schematic drawing of DHC-1, with the conserved domains indicated. C. elegans mutations are indicated above, and the mouse and human mutations are marked beneath the scheme. (c) Quantification of dendritic SVP accumulations in dhc-1 mutant alleles and transgenic rescue with genomic dhc-1 fragment. L4 and young adults were examined on a wide-field microscope, and any worm with a GFP::RAB-3 signal at the tip of the denrite was scored as positive. n=27–60, **P<0.01, χ2-test. (d) Subcellular localization of a rescuing GFP::DHC-1 transgene expressed specifically in DA9. (e) DLC-1::GFP is also enriched at the dendrite tip and can be detected at the axon tip. (f) In dhc-1(wy743) mutants, the distribution of DLC-1::GFP is similar to wt. (g) Dynein tip enrichment was quantified as the ratio between GFP::DHC-1 or DLC-1::GFP in the tip and the entire dendrite, and normalized to a soluble mCherry marker. n=17–23. H,I. Representative stall-force traces for wildtype (h) and SMA (i) dynein immunoprecipitated with a FLAG tag from COS-7 cells. (j) Plot of stall-force vs. stall time showing that SMA dynein is less processive than wildtype n=270 (control) and 220 (SMA) from two independent experiments. P<0.01 for the range between 0.2 and 0.6 s, ANOVA. Note that the range of forces observed is consistent with 1–2 motors. SA, splice acceptor.