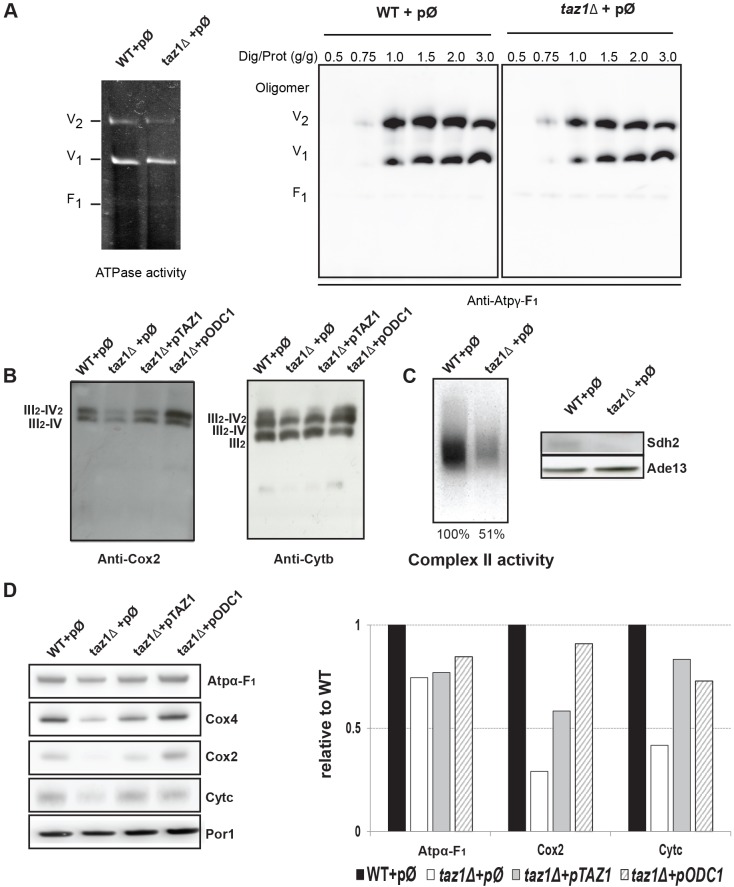
Fig. 5.
BN-PAGE and SDS-PAGE analyses of mitochondrial proteins. Experiments were performed with mitochondria isolated from strains WT+pØ, taz1Δ+ pØ, taz1Δ+pTAZ1 and taz1Δ+pODC1 grown as described in Fig. 2B. (A) BN-PAGE analyses of ATP synthase. The left panel shows a BN-gel of mitochondrial proteins (50 µg) dissolved with 2 g of digitonin per g of proteins, where ATP synthase is revealed by its ATPase activity as dimers (V2), monomers (V1) or free F1 particles (F1). In the right panel, ATP synthase was analyzed in samples (50 µg) obtained after treating the mitochondria with increasing concentrations of digitonin, from 0.5 to 3.0 g per g of protein. After their electrophoretic separation and transfer onto a nitrocellulose membrane, the proteins were probed with antibodies against the γ-F1 subunit (ATP3) of ATP synthase. (B) BN-PAGE analysis of CIV and CIII. Mitochondrial proteins were extracted with 10 g digitonin per g of protein, separated by BN-PAGE (100 µg per lane), transferred onto a nitrocellulose membrane, and probed with antibodies against the COX2 subunit of CIV or the cytochrome b subunit of CIII. (C) BN-PAGE and SDS-PAGE analyses of CII. On the left panel, mitochondrial proteins were extracted with digitonin (10 g/g), separated by BN-PAGE, and assayed for in-gel complex II activity; in the right panel, 100 µg of total protein extracts were separated by SDS-PAGE, transferred onto a nitrocellulose membrane and probed antibodies against SDH2 and ADE13. (D) SDS-PAGE analyses. 100 µg of total mitochondrial proteins were separated by SDS-PAGE, transferred onto a nitrocellulose membrane and probed with antibodies against the indicated proteins. The right panel shows a quantification which as been done using ImageJ. Levels of COX2, ATPα-F1 and cytochrome c are related to the mitochondrial protein Por1p. The data are all relative to WT.