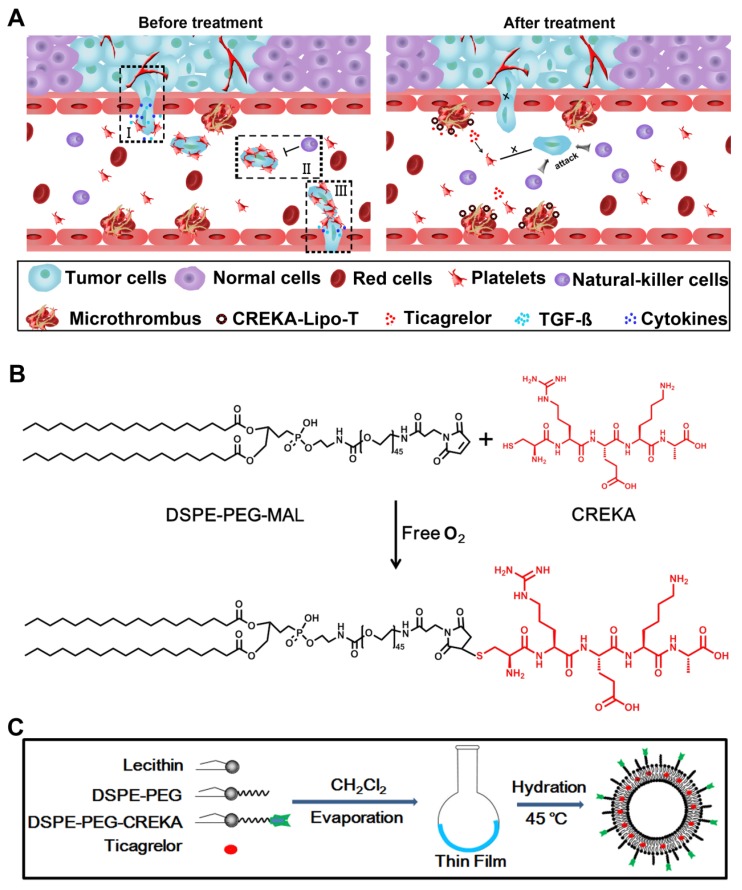
Figure 1.
The design of CREKA-Lipo-T nanoparticles and their proposed anti-metastatic mechanism within tumor tissues. (A) Proposed mechanism of action of CREKA-Lipo-T nanoparticles. Normally, TGF-ß secreted by platelets induce the tumor cells to transition to a mesenchymal-like phenotype (I). Platelets can also protect tumor cells against attack from NK cells (II). At distant sites, platelets assist the metastatic cells to cross the local endothelium by secreting many cytokines. Following treatment, CREKA-Lipo-T actively targets to microthrombi on the tumor vessel walls and releases ticagrelor slowly and locally. Ticagrelor binds to tumor-associated platelets and inhibits their functions. The release of TGF-ß from platelets and the interaction between platelets and tumor cells are abolished, leading to decreased epithelial-mesenchymal-like transition of the tumor cells, and thus inhibiting their invasion capacity. When the tumor cells are present in the circulation, the compromised platelets are unable to adhere to them and cannot shield them from NK cell attack. X indicates that a process was abolished by treatment. (B) The conjugation between DSPE-PEG and the CREKA peptide using the Michael addition reaction under anaerobic conditions. (C) Schematic diagram of the synthesis of CREKA-Lipo-T nanoparticles.