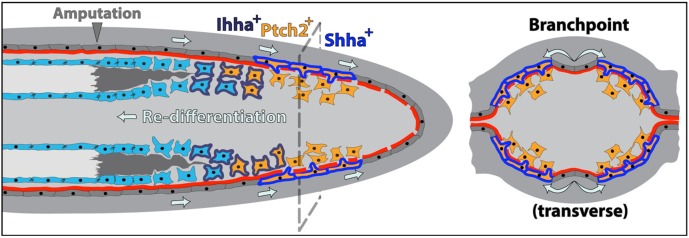
Fig. 8.
Model showing how cell movements, Hh/Smo pathway dynamics and direct cell-to-cell interactions between neighboring basal epidermal cells and progenitor osteoblasts induce ray bifurcation during fin regeneration. Basal epidermal cells generated proximal to the caudal fin amputation site continuously advance distally along a mature basal lamina. During this effective migration, groups of basal epidermal cells overlying the regenerating blastema transiently upregulate shha expression (cells outlined in blue) and then split into two clusters on each side of the ray. Shha drives active Hh/Smo signaling marked by ptch2 expression (orange cells) in a narrow distal zone of shha-expressing epidermal cells and adjacent progenitor osteoblasts (pObs). As fin outgrowth proceeds, some pObs escape self-renewal signals, initiate re-differentiation and progressively extend reforming bone. Fully differentiated Obs secrete new bone matrix, enabled by the earlier non-canonical activity of Ob-expressed Ihha (cells outlined in dark blue). The interface between Hh/Smo-responsive Obs and basal epidermis is the active site of basal lamina (red) assembly associated with fin regenerative outgrowth. Coinciding with the epidermal ‘branchpoint’, this incompletely assembled basement membrane enables shha-expressing basal epidermal cells to extend cellular protrusions that contact neighboring pObs. This direct binding activates Smo-dependent signaling (including further ptch2 expression) and progressively escorts pObs into physically separated pools. The newly divided pools of Runx2+ pObs then continue regenerating independently to produce a now bifurcated ray.