Figure 1.
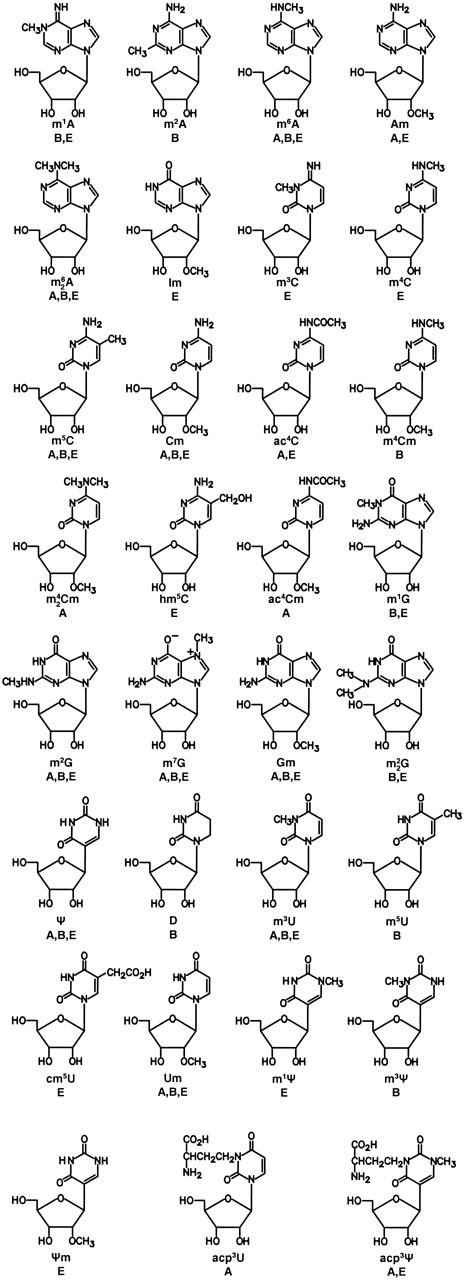
Chemical structures of presently known post-transcriptionally modified nucleosides from rRNA. A, archaea; B, bacteria; and E, eukarya. Symbols: m1A, 1-methyladenosine; m2A, 2-methyladenosine; m6A, N6-methyladenosine; Am, 2′-O-methyladenosine; m62A, N6,N6-dimethyladenosine; Im, 2′-O-methylinosine; m3C, 3-methylcytidine; m4C, N4-methlycytidine; m5C, 5-methlycytidine; Cm, 2′-O-methylcytidine; ac4C, N4-acetylcytidine; m4Cm, N4,2′-O-dimethylcytidine; m42Cm, N4,N4,2′-O-trimethylcytidine; hm5C, 5-hydroxymethylcytidine; ac4Cm, N4-acetyl-2′-O-methylcytidine; m1G, 1-methylguanosine; m2G, N2-methylguanosine; m7G, 7-methylguanosine; Gm, 2′-O-methylguanosine; m22G, N2,N2-dimethylguanosine; Ψ, pseudouridine; D, dihydrouridine; m3U, 3-methyluridine; m5U, 5-methyluridine; cm5U, 5-carboxymethyluridine; Um, 2′-O-methyluridine; m1Ψ, 1-methylpseudouridine; m3Ψ, 3-methylpseudouridine; Ψm, 2′-O-methylpseudouridine; acp3U, 3-(3-amino-3-carboxypropyl)uridine; and m1acp3Ψ, 1-methyl-3-(3-amino-3-carboxypropyl)pseudouridine.
