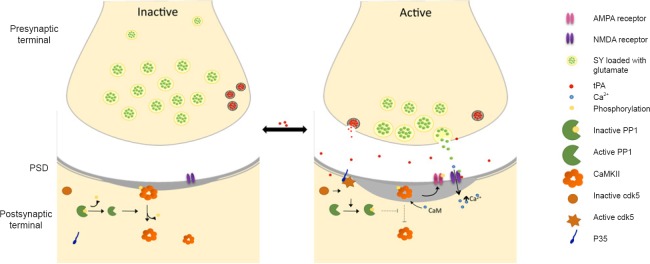
Figure 1.
Tissue-type plasminogen activator (tPA) induces homeostatic plasticity in cerebral cortical neurons.
tPA induces bidirectional changes in the structure and protein composition of the postsynaptic density (PSD) via its ability to regulate the protein phosphatases (PP) 1/Calmodulin-dependent protein kinase II (pCaMKII) switch. (A) In inactive neurons with low baseline expression of pCaMKIIa, tPA activates the kinase by inducing its phosphorylation at T286 in a Ca2+- and N-methyl-D-aspartate receptor (NMDAR)-dependent manner, followed by its subsequent translocation to the postsynaptic density where it induces the phosphorylation and synaptic recruitment of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, thus converting silent into active synapses. When activated by p35, cyclin-dependent kinase 5 (Cdk5) induces the PP1 phosphorylation at T320, an event that inactivates PP1 and thereby allows the phosphorylation and synaptic accumulation of pCaMKII. (B) In overactive neurons, tPA prevents p35-induced Cdk5 activation and Cdk5-induced PP1 inactivation by T320 phosphorylation. The active phosphatase is then able to dephosphorylate pCaMKIIa preventing its accumulation in the postsynaptic density (PSD) and abrogating the effect of pCaMKIIa on the synaptic recruitment of GluR1-containing AMPA receptors.