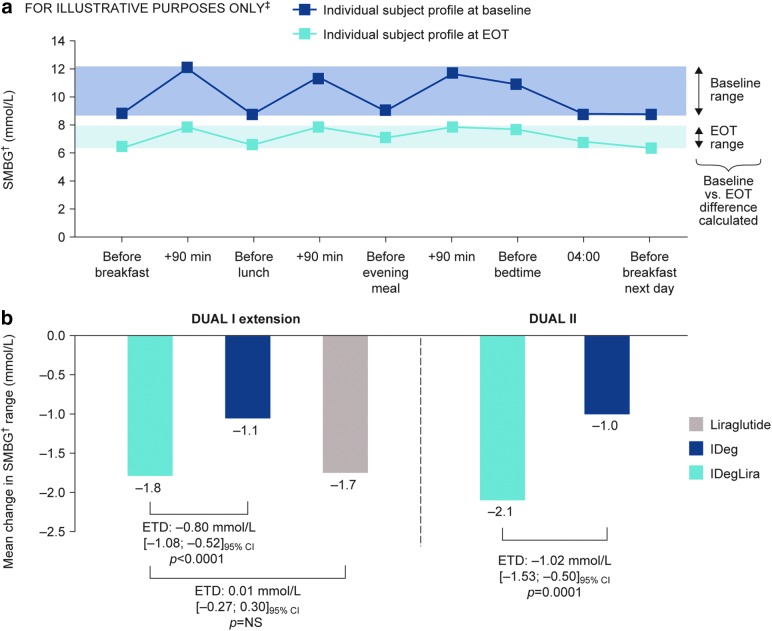
FIG. 1.
Nine-point SMBG† profile‡ showing calculation of range (a) and change from baseline to EOT in DUAL I and DUAL II (b). †SMBG assessed with glucose meter as plasma equivalent values of capillary whole blood glucose. ‡Illustrative example not intended to represent actual patients or treatment effects. Data are based on FAS, with LOCF for all subjects with a full nine-point profile at baseline; P-values are from ANCOVA with treatment, region, baseline HbA1c stratum (≤8.3% [≤67 mmol/mol], >8.3% [>67 mmol/mol]), and previous OAD treatment as fixed effects, and baseline value of the parameter included as covariate. ANCOVA, analysis of covariance; EOT, end-of-trial; ETD, estimated treatment difference; FAS, full analysis set; HbA1c, glycosylated hemoglobin; IDeg, insulin degludec; IDegLira, fixed ratio combination of insulin degludec and liraglutide; Lira, liraglutide; LOCF, last observation carried forward; OAD, oral antidiabetic drug; NS, not significant; SMBG, self-monitored blood glucose.