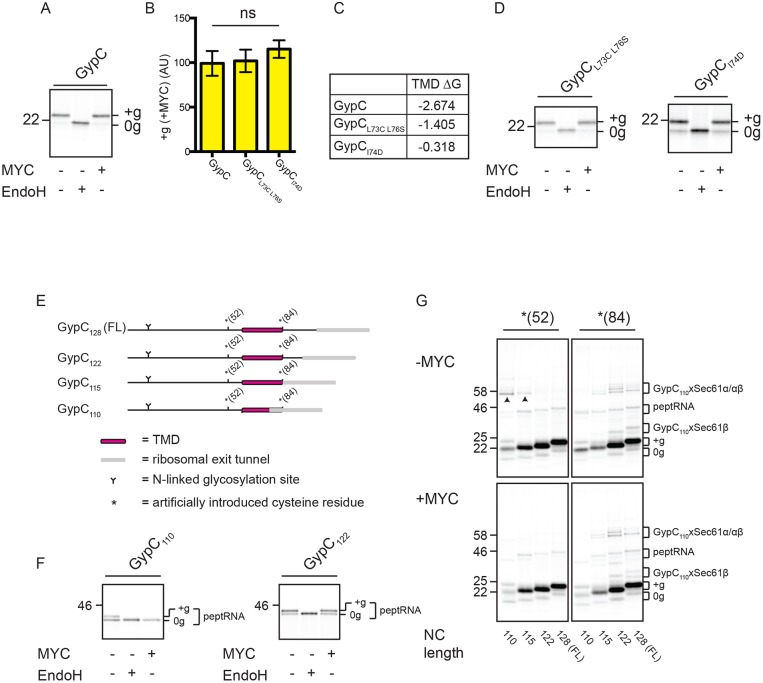
Fig. 4.
Mycolactone does not interfere with type III TMP integration. (A) Translation of GypC in the absence or presence of mycolactone (MYC), followed by subsequent treatment with EndoglycosidaseH (EndoH). (B) Graph shows change in the amount of glycosylated (+g) GypC and related constructs in the presence of mycolactone, relative to control samples as described in the legend to Fig. 1. The statistical test performed was one-way ANOVA. Error bars show mean±s.d. GypC, n=10; others, n=3. Ns, not significant. (C) Estimated TMD hydrophobicities (kcal/mol) of GypC and related constructs. (D) Translation of two variants of GypC with reduced TMD hydrophobicity. (E) GypC truncations lacking stop codons. For crosslinking experiments, truncations contained a single artificially introduced cysteine residue at either position 52 or 84, as denoted by an asterisk. (F) Truncated GypC chains synthesised in the absence or presence of mycolactone without puromycin-mediated release. The glycosylation of nascent chains when still attached to the ribosome (indicated by ‘peptRNA’) was observed. (G) Truncated GypC chains containing a single cysteine residue [either *(52) or *(84)] synthesised in the absence or presence of mycolactone without puromycin-mediated release to generate membrane integration intermediates. Samples were treated with the crosslinking reagent BMH, subjected to extraction with alkaline sodium carbonate, and analysed by SDS-PAGE. Adducts between the nascent chain and Sec61β (xSec61β) or the nascent chain and Sec61α/Sec61α and Sec61β (xSec61α/αβ) are indicated (see also Fig. S3B). Mycolactone-sensitive adducts are indicated by arrowheads. Other symbols are as defined in Fig. 1 legend. FL, full length.