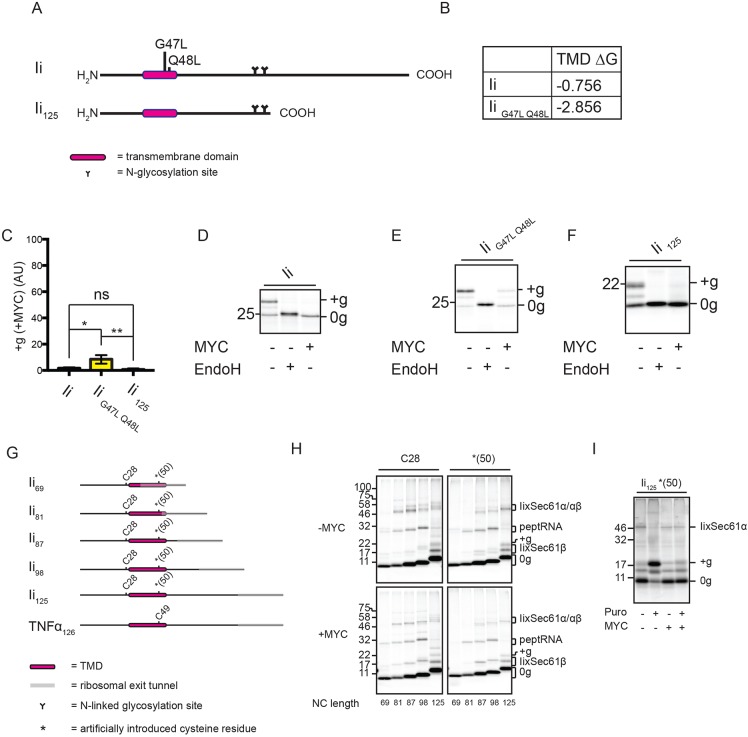
Fig. 5.
Mycolactone efficiently blocks type II TMP integration. (A) Full-length Ii (wild type and G47L Q48L mutant) and the Ii125 truncation used in this study. (B) Estimated TMD hydrophobicities (kcal/mol) of Ii and IiG47L Q48L. (C) Graph shows the reduction in the amount of glycosylated (+g) Ii and related constructs in the presence of mycolactone (MYC), relative to control samples as described in the legend to Fig. 1. The statistical test performed was one-way ANOVA. Error bars show mean±s.d. (n=3). P-values are as defined in Fig. 1 legend. Translation in the absence or presence of mycolactone performed using Ii (D), IiG47L Q48L (E) and Ii125 (F), which was followed by treatment with EndoglycosidaseH (EndoH). (G) Ii truncations used in this study. For crosslinking experiments, truncations contained either a native cysteine residue (C28) or one that was artificially introduced [*(50)]. A truncated version of TNFα used for crosslinking analysis (as described in MacKinnon et al., 2014) is shown for comparative purposes. Crosslinking was performed on Ii truncations (H) and Ii125*(50) (I) and the resulting adducts are labelled as described in the Fig. 4G legend. Other symbols are as defined in Fig. 1 legend. Puro, puromycin.