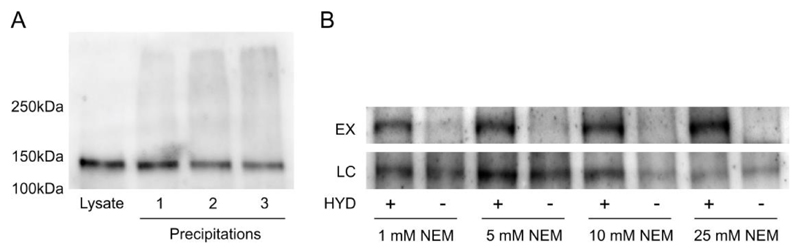
Figure 1.
(A) FLS2 aggregates and smears on 7.5% SDS-PAGE gels after multiple precipitations. 50µg of total protein was loaded per lane. FLS2 appears heavier than the predicted 130 kDa due to extensive glycosylation of the leucine-rich repeats. (B) Minimum concentrations of N-ethylmaleimide (NEM) required for elimination of false positive identification of FLS2 S-palmitoylation levels in acyl-biotin exchange assays. Acyl-biotin exchange assays were performed using the indicated concentrations of NEM during the blocking stage. All other steps in the protocol were as described in the methods. HYD indicates presence (+) or absence (-) of hydroxylamine required for acyl group cleavage during the biotin labelling step. Samples were bound to neutravidin beads following biotin labelling and enriched proteins were eluted and represent S-palmitoylated proteins (EX). Prior to neutravidin binding a sample was removed as an input loading control (LC). All samples were run on 7.5% SDS-PAGE gels and blotted for FLS2.