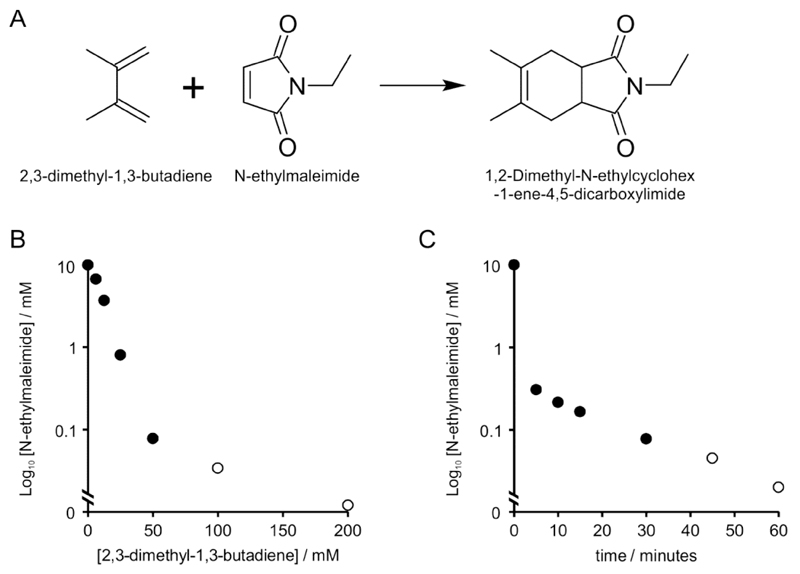
Figure 2.
(A) Reaction scheme of N-ethylmaleimide combining with 2,3-dimethyl-1,3-butadiene. (B) Determination of 2,3-dimethyl-1,3-butadiene concentration required for elimination of 10 mM N-ethylmaleimide (NEM) from lysis buffer in 1 hour. A standard curve of NEM was prepared in lysis buffer and absorbance at 300 nm measured. A 10mM solution of NEM was treated with a range of 2,3-dimethyl-1,3-butadiene concentrations for 1 hour at 25°C and remaining NEM concentration were determined from standard curves by spectrophotometric (filled circles) or fluorometric (open circles) methods. Data points are averages of 3 technical replicates. (C) Determination of time required for elimination of 10mM NEM by 100 mM 2,3-dimethyl-1,3-butadiene and chloroform extraction in lysis buffer. A standard curve of NEM was prepared in lysis buffer and absorbance at 300 nm measured. A 10 mM solution of NEM was treated with 100 mM 2,3-dimethyl-1,3-butadiene for the times indicated at 25°C and phase partitioned using chloroform. Remaining NEM concentration were determined from standard curves by spectrophotometric (filled circles) or fluorometric (open circles) methods. Data points are averages of 3 technical replicates.