Figure 2.
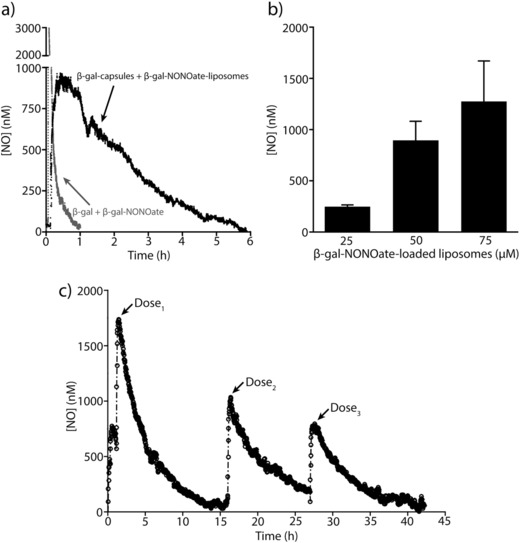
Nitric oxide (NO) release from β‐galactosidase encapsulated in capsules and β‐gal‐NONOate loaded in liposomes. a) Representative NO decay curve from enzymatic hydrolysis of β‐gal‐NONOate from liposomes (50 × 10−6 m, 106 liposomes µL−1) by β‐galactosidase encapsulated in capsules (106 capsules µL−1) or enzymatic hydrolysis of non‐encapsulated β‐gal‐NONOate and β‐galactosidase in DBG solution at 37 °C. By spatially separating enzymes and NO donors into polymer capsules and liposomes, respectively, the half‐life of NO released increased significantly, extending the t 1/2 from ≈5 min to ≈2.5 h. A maximal NO concentration of ≈900 × 10−9 m was generated in 30 min. b) Maximal NO concentration generated in 30 min as a function of increasing loading concentration of β‐gal‐NONOate encapsulated inside the liposomes (25 × 10−6 to 75 × 10−6 m β‐gal‐NONOate, 106 liposomes µL−1), which were added into a suspension of capsules containing β‐galactosidase (106 capsules µL−1) in DBG solution at 37 °C (n = 3 for each concentration; error bars are SD). c) Sustained release of NO from β‐galactosidase encapsulated in PMA capsules and β‐gal‐NONOate loaded in liposomes. Representative NO decay curve from enzymatic hydrolysis of β‐gal‐NONOate from liposomes (75 × 10−6 m, 106 liposomes µL−1) by β‐galactosidase encapsulated in capsules (106 capsules µL−1) in DBG solution at 37 °C is shown. The enzymatic catalysis was repeated over three cycles by removing the hydrolyzed substrates and adding fresh β‐gal‐NONOate‐loaded liposomes into the suspension of capsules containing β‐galactosidase. Over 42 h the enzyme retained 50% of its activity.
