Abstract
Aims
Mechanical stimulation (MS) represents a readily available, non-invasive means of pacing the asystolic or bradycardic heart in patients, but benefits of MS at higher heart rates are unclear. Our aim was to assess the maximum rate and sustainability of excitation by MS vs. electrical stimulation (ES) in the isolated heart under normal physiological conditions.
Methods and results
Trains of local MS or ES at rates exceeding intrinsic sinus rhythm (overdrive pacing; lowest pacing rates 2.5±0.5 Hz) were applied to the same mid-left ventricular free-wall site on the epicardium of Langendorff-perfused rabbit hearts. Stimulation rates were progressively increased, with a recovery period of normal sinus rhythm between each stimulation period. Trains of MS caused repeated focal ventricular excitation from the site of stimulation. The maximum rate at which MS achieved 1:1 capture was lower than during ES (4.2±0.2 vs. 5.9±0.2 Hz, respectively). At all overdrive pacing rates for which repetitive MS was possible, 1:1 capture was reversibly lost after a finite number of cycles, even though same-site capture by ES remained possible. The number of MS cycles until loss of capture decreased with rising stimulation rate. If interspersed with ES, the number of MS to failure of capture was lower than for MS only.
Conclusion
In this study, we demonstrate that the maximum pacing rate at which MS can be sustained is lower than that for same-site ES in isolated heart, and that, in contrast to ES, the sustainability of successful 1:1 capture by MS is limited. The mechanism(s) of differences in MS vs. ES pacing ability, potentially important for emergency heart rhythm management, are currently unknown, thus warranting further investigation.
Keywords: Cardiac , Electrophysiology , Mechano-electric feedback , Optical mapping , Stretch-activated channels , Strain
What’s new?
Localised non-contusional mechanical stimulation can pace the ventricles in isolated whole-heart.
Maximum pacing rates achievable with mechanical stimulation are lower than with electrical stimulation.
Pacing capture by mechanical stimulation at rates exceeding normal sinus rhythm is reversibly lost after a finite number of cycles; this number decreases with increasing pacing rate, even though the stimulated tissue remains electrically excitable.
When interspersed with electrical stimuli, the number of mechanically-paced beats to loss of capture is lower than with same-frequency mechanical stimulation only.
This suggests the presence of a ‘depletable pool of mediator(s)’ required for mechano-electric coupling, which is also sensitive to electrical stimulation.
The nature of the ‘mediator(s)’ is currently unknown.
Introduction
While implanted electrical pacemakers are an effective means of sustained cardiac pacing, mechanical pacing is of interest for the emergency resuscitation setting, as it represents a rapidly available, non-invasive and generally well-tolerated method for triggering cardiac excitation in the asystolic or severely bradycardic heart.1
Localized mechanical stimulation (MS) of the human heart, whether by direct tissue contact of intra-cardiac devices (catheters, pacing leads)2 or by extracorporeal impact (Commotio cordis,3 precordial thump4) can cause mechanically-induced ventricular excitation (VEM), resulting in competent ventricular contraction. If applied rhythmically to the precordium (‘precordial percussion’),5 repetitive thumps have been shown to be an effective means for extracorporeal pacing of the asystolic6,7 or bradycardic8 heart, while benefits of MS for cardioversion of tachycardias is limited.9 As mechanically-induced heartbeats have a greater hemodynamic effect than external chest compressions,10 they can maintain consciousness in patients during extended periods of ventricular standstill (cases as long as 2 h 45 min have been reported).6,11
This ‘mechano-electric feedback’ effect12 was exploited by resuscitation pioneer Paul Zoll in the design of a mechanical pacing device for external stimulation of heart beats in emergency settings (‘cardiac thumper’).13 In dogs with normal sinus rhythm and high-degree atrioventricular block, repetitive heartbeats were evoked using this device, with no cases of mechanically-induced sustained arrhythmia (such as tachycardia or fibrillation, which can occur following VEM14), even when stimulation occurred during the relative refractory period and at energies up to 10 times VEM threshold. The device was also shown to be effective in patients with asystole after ventricular fibrillation, with atrial fibrillation, or with implanted pacemakers for atrioventricular block.13
More recent device-based mechanical pacing efforts have focused on the use of extracorporeal high intensity focused ultrasound,15 which has been shown to excite frog,16 mouse,17 rat,18 and pig19 hearts, and intravenously injected magnetic microparticles manipulated by an external electromagnet, which can mechanically pace the right ventricle of rat and pig.20 Maximum rates and sustainability of repetitive mechanical pacing, however, have remained ill-explored.
Using optical mapping of direct local MS of the ventricular epicardium in rat21 and rabbit22 isolated hearts, we have shown that VEM originates focally from the stimulation site, spreading downstream from the point of earliest activation in a manner that is indistinguishable from electrically paced beats (including parameters such as dVm/dtmax, action potential duration, conduction velocity). Mechanical pacing occurs by activation of cation-non-selective stretch-activated channels (SACNS),23 as it is prevented by SACNS block with Grammostola spatulata MechanoToxin-4 (GsMTx-4).22 In terms of macroscopic mechanics, VEM depends on the extent of local tissue indentation.22 Whether repetitive supra-threshold mechanical stimuli can continuously cause excitation in the spontaneously-active isolated heart, and if so, at what maximum rate, is unknown. The goal of this study, therefore, was to investigate the maximum rate and sustainability of local VEM induction, compared to same-site electrical stimulation (ES), in the isolated Langendorff-perfused rabbit heart.
Methods
Ethical approval
This study was carried out, with local ethical approval, in accordance with the UK Home Office Animals (Scientific Procedures) Act of 1986. Details of experimental protocols have been reported following the Minimum Information about a Cardiac Electrophysiology Experiment (MICEE) reporting standard,24 see online repository (https://www.micee.org/?q=node/00001378).
Heart preparation
Female New Zealand White rabbits (1.4 ± 0.3 kg) were euthanized by ear vein injection of 140 mg/kg pentobarbital. After thoracotomy, the heart was swiftly excised and placed in Krebs–Henseleit solution (containing [in mM]: 120 NaCI; 4.7 KCl; 24 NaHCO3; 1.4 NaH2PO4; 1.0 MgCl2, 1.8 CaCl2; 10 glucose; osmolality: 300 ± 5 mOsm/kg; pH: 7.4 ± 0.05) bubbled with carbogen (95% O2, 5% CO2). The heart was connected rapidly (1–2 min from excision) and bubble-free to a custom Langendorff apparatus by aortic cannulation, and perfused with 37°C Krebs–Henseleit solution at 15 mL/min. Perfusion pressure was monitored with a transducer (TSD104A; Biopac Systems Inc., Goleta, CA), and temperature with a fast-response thermistor (TSD202A; Biopac Systems Inc.) positioned in the aortic cannula via a three-way stopcock. An incision into the proximal pulmonary artery allowed coronary effluent to exit the right ventricle. Remaining extra-cardiac tissue (lungs, thymus, pericardium, vessels) was removed. For mechanical support during epicardial MS, the heart was positioned into an individually pre-molded Parafilm (Bemis Company Inc., Oshkosh, WI) cradle with black backing, and the entire perfusion system was angled at 45°, with the left ventricle (LV) facing upwards, to allow surface-perpendicular mechanical contact during optical measurements. The exposed epicardial surface was superfused with warm Krebs–Henseleit solution at a rate of 1 mL/min.
A 4–5 mm incision was made in the mid-left atrial auriculum. A short piece of 18 G intravenous cannula was passed through the incision, across the mitral orifice into the LV and pushed transmurally though the apex to prevent intra-ventricular fluid build-up. A custom-made pre-strained deflated polyethylene balloon, fitted on a 10 mm piece of manometer line (2 mm inner diameter) filled with degassed water and connected to a three-way stopcock, was inserted into the LV via the auricular incision. The balloon tip was secured at the ventricular apex by a 3–0 silk suture through the apical cannula. The atrium was tied to the manometer line by a silk ligature to secure the base of the balloon inside the LV. Intra-ventricular pressure was monitored with a transducer (TSD104A; Biopac Systems Inc.) connected to the balloon stopcock. The balloon, pressure transducer and connections were kept air-free to prevent damping of the pressure signal. A surface ECG was measured using two spring-loaded monopolar Ag/AgCl pellet electrodes (PY2 73-0200; Harvard Apparatus, Holliston, MA), one contacting the right atrium and the other the LV apex. Temperature, perfusion pressure and ECG sensors were interfaced with a data acquisition system (MP150; Biopac Systems Inc.) and data were collected at 2 kHz. After a 15 min equilibration period, the intra-ventricular balloon was inflated with degassed water to a diastolic pressure of 0–5 mmHg. The experimental setup can be seen in Figure 1.
Figure 1.
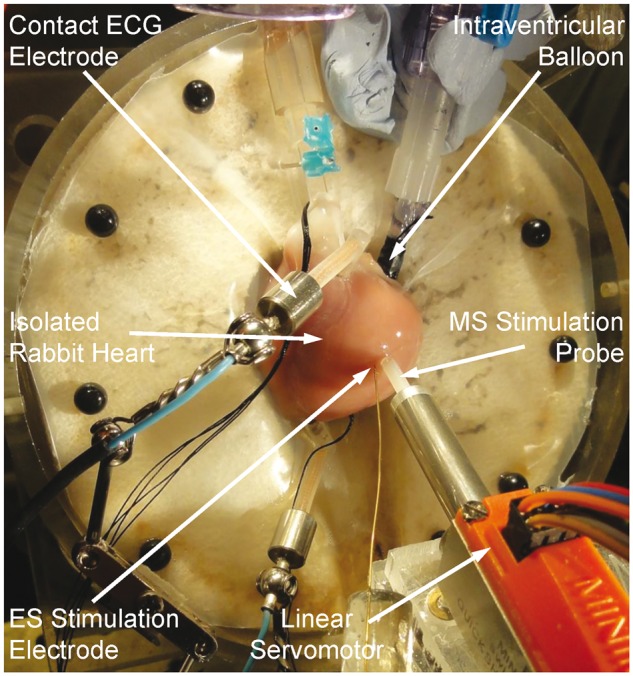
Photographic image of the experimental setup, showing an isolated rabbit heart instrumented with contact electrocardiogram (ECG) electrodes, an intraventricular balloon, electrical stimulation (ES) electrode and mechanical stimulation (MS) probe, coupled to a linear servomotor.
Mechanical and electrical stimulation
Comparison of MS and ES was performed on n = 6 spontaneously active healthy hearts. Local MS was applied at the mid-LV free-wall by epicardial indentation with a 3.1 mm2 contact-area probe. This was accomplished by swift forward and reverse motion of the probe by a computer-controlled linear DC-servomotor (LM 1247-02-01; Faulhaber MiniMotor SA, Croglio, Switzerland) regulated by a motion controller with position decoder (MCLM 2006 S; Faulhaber MiniMotor SA) using custom programs developed in Motion Manager (Faulhaber MiniMotor SA). In an initial series of additional hearts (n = 3), the degree of tissue indentation needed to ensure reliable induction of excitation was assessed. In agreement with previous results (in n = 7 separate hearts), the threshold for VEM (at an indentation rate of 300 mm/s) was ∼2 mm indentation.22 Thus to ensure reliable induction of VEM, a 3 mm indentation depth was chosen for MS (1.5× threshold), with indentation and retraction each occurring over 10 ms.
Local ES (2 ms bipolar pulse, with voltage set to 1.5× threshold, generally ∼3 V) was applied at the center of the same mid-LV free-wall location using a point (100 μm diameter) concentric bipolar stimulation electrode (SNE-100; Lohmann Research, Castrop-Rauxel, Germany).
Trains of 200 MS or ES were applied at stimulation rates that increased from 0.5 Hz above intrinsic sinus rate to 6.5 Hz, in 0.5 Hz increments, with a 1 min recovery period of normal sinus rhythm between each stimulation period. Timing relative to the cardiac cycle of the first stimulus in each train was controlled by triggering from the peak of the ECG R-wave to be diastolic using custom-designed electronics and programs developed in MATLAB (MathWorks, Natick, MA).
To evaluate the focal nature of excitation, following the MS/ES train series, hearts were loaded with a voltage-sensitive fluorescent dye (20 μL bolus of 27.3 mM di-4-ANBDQPQ solution in medical grade ethanol injected directly into the aortic cannula in 0.4 μL increments over 2 min, i.e. diluted in 30 mL of perfusate to an effective concentration of 23.4 μM; dye acquired from the University of Connecticut Health Center, Farmington, CT). Optical mapping was performed as described previously.21 Briefly, fluorescence was excited by two red light-emitting diodes (CBT-90-R; Luminus Devices Inc., Billerica, MA) through band-pass filters (D640/20X; Chroma Technology Corp., Bellows Falls, VT). Emission was collected with a 50 mm high-speed lens (DO-5095; Navitar, Rochester, NY) through a long-pass filter (HQ690LP Chroma Technology Corp.) and recorded at 511 frames per second by a 128 × 128 pixel, 16-bit electron multiplying charge coupled device camera (Cascade: 128+; Photometrics, Tucson, AZ) controlled using MultiRecorder (developed by Stefan Luther and Johannes Schröder-Schetelig, Max Planck Institute for Dynamics and Self-Organization, Göttingen, Germany).
To evaluate whether MS was indeed sub-contusional, tissue integrity was assessed by analysis of creatine kinase activity in coronary effluent (17296H CK-NAC Liquid; Alpha Laboratories Ltd., Eastleigh, United Kingdom) using a spectrophotometer (BioTech UV1101; Biochrom WPA, Cambridge, United Kingdom), which has been previously shown to reliably track tissue damage associated with contusional MS in rabbit isolated hearts.25
Data analysis
Data were analyzed using custom programs in Matlab (MathWorks). Values are presented as mean ± standard error, with means compared by two-tailed, paired Student’s t-test, with a P value < 0.05 indicating a statistically significant difference between means.
Results
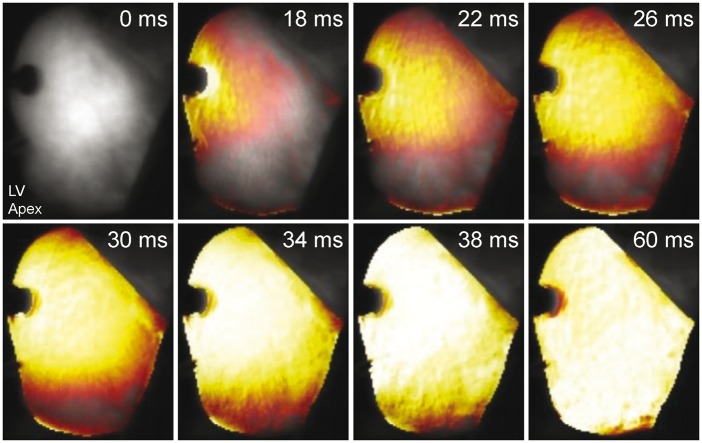
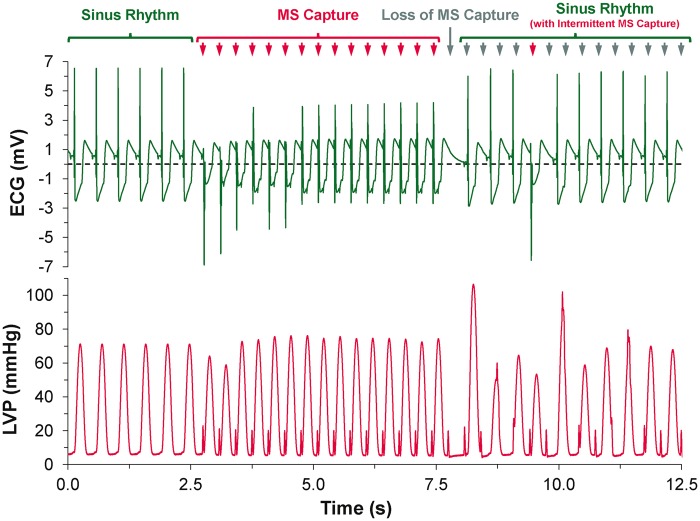
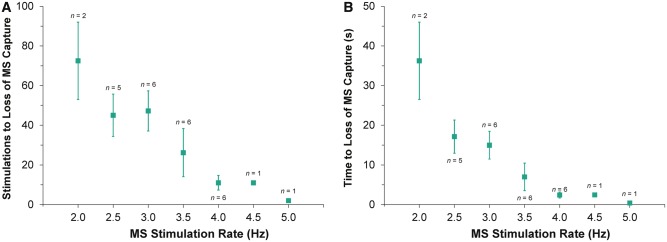
Supra-threshold MS reliably caused focal excitation from the site of stimulation (Figure 2, see Supplementary material online, Movie S1). With repetitive stimulation at rates exceeding spontaneous pacemaker activity (overdrive pacing), the maximum rate of stimulation at which 1:1 capture occurred was lower for MS than for ES in all hearts (4.2 ± 0.2 vs. 5.9 ± 0.2 Hz; P = 0.001). For each overdrive pacing rate with 1:1 MS capture (i.e. at stimulation rates between 2.5 ± 0.5 Hz and 4.5 ± 0.5 Hz), ES was sustained for the entire 200-cycle stimulation period. With MS, however, capture was lost during the period of stimulation (Figure 3), after a finite number of stimuli. The number of consecutive MS with 1:1 capture, and the duration of 1:1 capture, decreased with increasing stimulation rate (Figure 4). The loss of capture with MS was reversible, as VEM re-occurred after a 1 min recovery period of spontaneous sinus rhythm. During MS failure, there was no general loss of LV excitability, as ES applied to the same LV tissue site was still able to excite the heart.
Figure 2.
Mechanically-induced focal excitation of the left ventricle visualised by epicardial optical mapping (frames taken from Movie S1; see Supplementary material online).
Figure 3.
Electrocardiogram (ECG) and left ventricular pressure (LVP) recordings during sinus rhythm, followed by a train of mechanical stimulations (MS) at a rate of 3 Hz during which loss of 1:1 capture occurs, resulting in a return to sinus rhythm with intermittent MS capture.
Figure 4.
Effect of overdrive pacing rate by mechanical stimulation (MS) on the number of stimulations (A) and time (B) to loss of MS 1:1 capture.
There was no tissue damage, as confirmed by a lack of creatine kinase increase in the coronary effluent, the absence of changes in either heart rate or ECG configuration during return to normal sinus rhythm, and the ability to resume same-site MS after a 1 min pause.
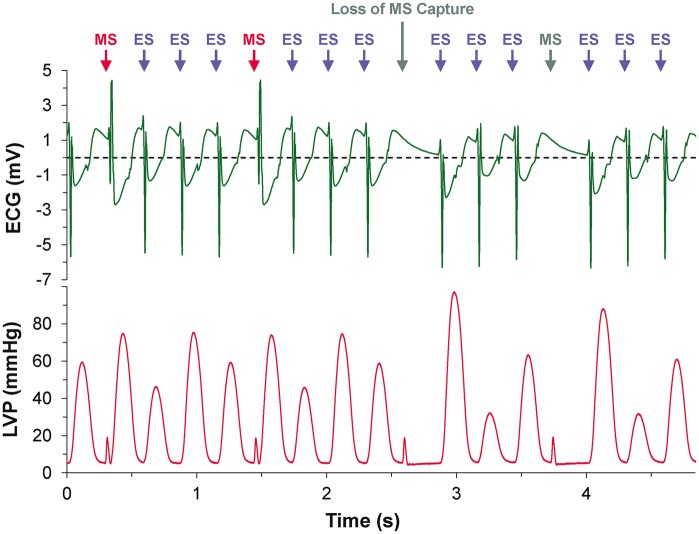
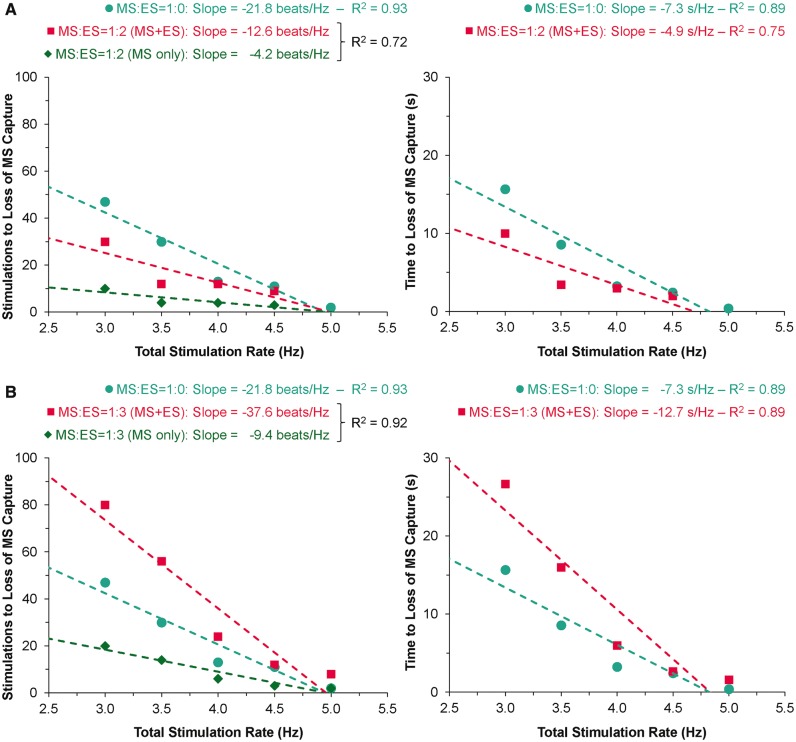
To further investigate the relation of mechanical and electrical capture, series of MS were interspersed with ES (thus reducing the effective MS application rate, while maintaining total pacing rate; Figure 5). Counterintuitively, at MS:ES ratios of 1:1 and 1:2, there was a decrease in the total number of stimulations (combined MS and ES) and time to loss of capture by MS (Figure 6A). At further decreased relative contributions of MS (MS:ES ratios of 1:3 or more), the total number of stimulations (MS and ES combined) and time to loss of capture by MS were increased (Figure 6B). However, for all MS:ES trains, the number of MS that could be applied before a loss of capture occurred was reduced by comparison with trains consisting of MS only (green diamonds vs. blue circles in Figure 6). Capture by ES was maintained throughout all protocols (Figure 5).
Figure 5.
Electrocardiogram (ECG) and left ventricular pressure (LVP) recordings during a train of 1:3 mechanical:electrical (MS:ES) stimulations at a total stimulation rate of 3 Hz during which loss of MS capture occurs after 2.5 s, while ES capture is maintained.
Figure 6.
Effect of total stimulation rate on the number of total (mechanical + electrical, MS + ES) and MS-only stimulations (left) and time (right) to loss of MS 1:1 capture for trains of MS only (1:0) and mixed MS:ES at ratios of 1:2 (A) or 1:3 (B). Note that the green diamonds show the number of MS during MS:ES trains (net rate of MS is reduced by interspersed ES). The dashed lines were generated by linear regression.
Discussion
This study sought to compare maximum rates and sustainability of MS vs. ES in isolated Langendorff-perfused rabbit hearts. Repetitive local MS of the LV epicardium causes repeated focal VEM. The maximum rate at which stimulation is able to achieve 1:1 capture is lower for MS than for ES. At all overdrive pacing rates for which repetitive MS is possible (roughly 2–5 Hz), 1:1 capture is reversibly lost after a finite number of stimulations, which may account for the loss of capture seen with continuous focused ultrasound15 and magnetic microparticle-based pacing.20 At the same time, the tissue remains electrically excitable, as capture by ES is maintained. The number of MS that can be applied until loss of capture decreases with rising stimulation rate. Surprisingly, if interspersed with ES, the maximum number of MS to failure of capture is lower than for same-frequency MS-only pacing.
Potential mechanisms
The findings that MS and ES allow different maximum pacing rates and that upon loss of MS capture excitation is still possible by ES suggest that MS and ES are subject to different types of (mechanical and/or electrical) ‘refractoriness’. This is supported by a study of repetitive local stimulation of LV epicardium in open-chest anesthetised dogs that demonstrated a decrease in the effective refractory period at the site of stimulation with ES (at a pacing rate of 2.5 Hz), but showed no change with MS, which could bias maximum achievable pacing rates in favour of ES.26 In a study employing transient inflation of an intra-ventricular balloon for MS (which has been described as an effective means for pacing the asystolic heart),27 repeat inflations were effective only after periods of rest (up to 1 min for full recovery of mechanically-induced excitability).28 Overall, these studies suggest that MS involves a ‘depletable yet replenishable’ pool of mechano-electric ‘mediator(s)’ that is different from established electrical mechanisms of refractoriness.
Potential mechanisms for a mechano-electric adaptation period, during which there is a temporary reduction of VEM-inducibility that returns after a period of normal sinus rhythm (demonstrated in this and previous reports), as well as the difference in maximum stimulation rates for MS and ES (shown here), include MS-specific effects on: (i) tissue mechanical properties (passive or viscoelastic); (ii) cytoskeletal elements; (iii) SACNS (or other ion channel activity); (iv) ionic distributions and/or availability; (v) intracellular (such as sarcoplasmic reticulum, mitochondria or lysosomes) or sarcolemmal domains (e.g. caveolae); (vi) second messenger systems that influence the above; or (vii) other unknown factors necessary for MS.
In theory, effects of repetitive mechanical stimulation on the mechanical properties of cardiac tissue could be an important mechanism involved in the use-dependent loss of MS-related excitability. For instance, during mechanical testing of passive whole hearts, ‘strain softening’ has been reported, by which tissue is stiffer for an initial deformation cycle compared to subsequent cycles, even after several hours of rest (distinguishing it from a viscoelastic effect).29 As MS in our experiments regained effectiveness in causing 1:1 capture after a 1 min period of normal sinus rhythm, strain softening is unlikely to be a mechanism. More importantly, while strain softening is evident in non-viable (electrically inexcitable) myocardium, it is not observed in viable preparations undergoing physiologically relevant deformation, even in the presence of 2,3-butanedione monoxime (which inhibits cross-bridge formation, thus excluding an active mechanical component).30 In viable samples, there is, however, a reversible decrease of muscle stiffness that occurs between the first and subsequent deformation cycles, requiring ∼30 s of rest to recover. This viscoelastic effect could potentially account for a mechano-electric adaptation period in our (and others’) experiments, which is further supported by the observed stimulation rate-dependent decrease in the number of MS before a loss of capture. However, the fact that mixed MS:ES trains resulted in loss of VEM-induction with fewer MS than MS-only trains and at a reduced effective rate of MS, makes this explanation unlikely. Moreover, the fact that different MS:ES ratios ( ≤1:2 vs. ≥1:3) had different effects on the total number of stimulations and time to loss of MS capture, and the observation that changes in the time to loss of MS capture did not scale with the change in MS rate, seems to indicate that mediators of MS and ES are in fact (at least partially) overlapping (which would again make a viscoelastic effect a less likely mechanism).
There is evidence to support the idea that SACNS (or other ion channels) show ‘mechanical refractoriness’. For SACNS, repetitive MS has been shown to cause a decrease in activated ion current. For instance, in acutely isolated embryonic chick heart cells, repeated MS causes a reduction in measured SACNS current, unless subsequent MS are spaced minutes apart.31 This use-dependent decrease in SACNS current could be responsible, in part at least, for the rate-dependent decrease in the number of MS to a loss of capture with repetitive MS seen in our experiments. At the same time, the ‘run-down’ of MS-inducible current is particularly pronounced after the first MS, with progressively less prominent reductions in SACNS current, which does not explain the faster loss of MS capture if interspersed with ES.
Mechanical effects on ionic concentrations, especially via modulation of sub-cellular compartments and/or sarcolemmal structures, may be another contributor to a loss of mechanical excitability. For instance, stretch has been shown to directly affect intracellular calcium handling in cardiac cells,32 including acute increases in localised sarcoplasmic reticulum calcium release events (calcium sparks) in ventricular myocytes,33 which reduces sarcoplasmic reticulum calcium levels. If alterations in calcium handling (such stretch-induced calcium release) are involved in VEM, then a depletion in sarcoplasmic reticulum calcium stores in general, or of a mechanically releasable sub-pool in particular, could affect MS effectiveness. An increase in calcium spark rate with stretch is thought to result either from direct mechanical stimulation of ryanodine receptor channels,33 or via effects mediated by reactive oxygen species,34 with microtubules acting as potential mechano-conductors. Both mechanisms could be affected by the frequency of cyclic MS, which could explain the stimulation rate-dependent decrease in the number of MS before a loss of capture in our experiments. Other sub-cellular compartments could include mechanically-induced calcium release from mitochondria (whose intra-organelle calcium concentrations may be similarly affected by stretch),35 or acidic stores (e.g. lysosomes).36 Equally, mechanical effects on membrane domains, such as stretch-induced incorporation of caveolae into the sarcolemma,37 have been reported and may play roles in electrical responses to MS.38,39
Why MS trains interspersed with ES should be accompanied by an accelerated loss of VEM-inducibility is not obvious, though, from any of the above candidate mechanisms.
Future directions
Defining the mechanisms responsible for differences in the maximum rate and sustainability of pacing with MS compared to ES requires further study. To determine the relative contributions of potential mechanisms (altered ion channel activity, ionic concentrations, intracellular or sarcolemmal domains and tissue mechanics) experiments should involve: (i) pharmacological modulation of relevant ion fluxes and/or sub-cellular structures; (ii) domain-specific modulation or reporting of changes in ion concentrations and/or buffering; (iii) alteration of extracellular biophysical properties, from composition of solutions to background ventricular pressure/volume load or material stiffness (cytoskeletal disruption and cross-bridge inhibition); and (iv) variation of MS characteristics (indentation magnitude and rate, force and contact area/pressure under the probe), combined with measurement of strain and force (preliminary results have shown an inter-dependence of VEM on indentation magnitude and rate, Figure 7) to assess, for example, whether MS at the point of failed 1:1 capture may still initiate VEM if using higher mechanical stimulus intensities (‘relative refractoriness’). Additional key experiments involve work at various levels of structural integration, for example to determine maximum rates and sustainability of MS and ES in single isolated cardiomyocytes, or of the effects of non-myocytes40 on cardiomyocyte responses to MS and ES in cell culture41 or tissue slices,42 combined with quantitative computational integration of findings.43 Of further interest would be investigations into various pathophysiological states to determine the influence of disease background, and in multiple species, to test for conservation of effects. For applied research, it would be pertinent to determine, the lowest MS rates that allow sustenance of 1:1 capture, for instance during AV block, and to compare MS at multiple sites in the same heart, to assess ‘regionality’ of mechanical effects and to explore whether alternating MS between multiple sites could be an effective means of sustaining long-term mechanical pacing. This would also allow one to determine if, in contrast to the present results, previously-reported sustenance of MS in patients5–8,11 is related to differences in pacing rate (below vs. above sinus rate), experimental preparation (in situ vs. ex situ), species (human vs. rabbit), MS delivery (extracorporeal vs. epicardial), MS characteristics (such as magnitude and/or rate), and/or other factors.
Figure 7.
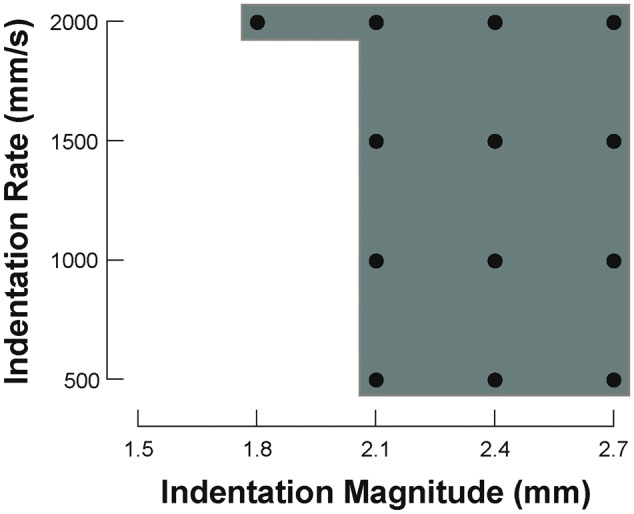
Inter-dependence of mechanically-induced excitation on indentation magnitude and rate. • indicates combinations of mechanical stimulation characteristics that elicited excitation.
Conclusions
The use of MS for cardiac pacing at comparatively low rates in acutely asystolic hearts deserves consideration as a rapidly-applicable means for emergency heart rhythm management. Primary asystole/bradycardia can develop with cardiac arrest, atrioventricular block, or after electrical defibrillation, and mechanical pacing may be effective as a bridge to instrumentation-based approaches, in particular in out-of-hospital and emergency settings. This requires a thorough understanding of the mechanisms and limitations of MS. Here we show that the maximum rate at which MS can be used for same-site LV pacing is lower than that for ES, and that with MS overdrive pacing there is a rate-dependent decrease in the number of MS to a loss of capture. The mechanism(s) of differences in the ability of MS vs. ES to pace the heart are currently unknown, warranting further investigation.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
This study is closely linked to the ‘TRM Forum’ meeting series at Lugano: reported at the meeting in 2015, the study was triggered by our inability to answer a question from Dr. Flavio Fenton (regarding maximum sustainability of electrical and mechanical pacing) during the 2011 edition. We are thankful to the meeting organisers and Dr. Fenton for stimulating discussions on this and other topics.
Funding
This work was supported by the Engineering and Physical Sciences Research Council [EPSRC, EP/F042868/1 to T.A.Q.]; the British Heart Foundation [BHF, PG/09/066 to T.A.Q.; FS/12/17/29532 to P.K.]; the European Research Council [ERC, Advanced Grant CardioNECT to P.K.]; and the Magdi Yacoub Institute. T.A.Q. was an EPSRC Postdoctoral Fellow; P.K. is a BHF Senior Fellow.
Conflict of interest: none declared.
References
- 1. Schott E. On ventricular standstill (Adam-Stokes attacks) together with other arrhythmias of temporary nature. Deutsches Archiv klinischer Medizin 1920;131:211–29. [Google Scholar]
- 2. Befeler B. Mechanical stimulation of the heart: its therapeutic value in tachyarrhythmias. Chest 1978;73:832–8. [DOI] [PubMed] [Google Scholar]
- 3. Kohl P, Nesbitt AD, Cooper PJ, Lei M. Sudden cardiac death by Commotio cordis: role of mechano-electric feedback. Cardiovasc Res 2001;50:280–9. [DOI] [PubMed] [Google Scholar]
- 4. Monteleone PP, Alibertis K, Brady WJ. Emergent precordial percussion revisited—pacing the heart in asystole. Am J Emerg Med 2011;29:563–5. [DOI] [PubMed] [Google Scholar]
- 5. Scherf D, Bornemann C. Thumping of the precordium in ventricular standstill. Am J Cardiol 1960;5:30–40. [DOI] [PubMed] [Google Scholar]
- 6. Michael TA, Stanford RL. Precordial percussion in cardiac asystole. Lancet 1963;1:699. [DOI] [PubMed] [Google Scholar]
- 7. Madias C, Maron BJ, Alsheikh-Ali AA, Rajab M, Estes NA, III, Link MS. Precordial thump for cardiac arrest is effective for asystole but not for ventricular fibrillation. Heart Rhythm 2009;6:1495–500. [DOI] [PubMed] [Google Scholar]
- 8. Eich C, Bleckmann A, Schwarz SK. Percussion pacing–an almost forgotten procedure for haemodynamically unstable bradycardias? A report of three case studies and review of the literature. Br J Anaesth 2007;98:429–33. [DOI] [PubMed] [Google Scholar]
- 9. Pellis T, Kette F, Lovisa D, Franceschino E, Magagnin L, Mercante WP. et al. Utility of pre-cordial thump for treatment of out of hospital cardiac arrest: a prospective study. Resuscitation 2009;80:17–23. [DOI] [PubMed] [Google Scholar]
- 10. Phillips JH, Burch GE. Management of cardiac arrest. Am Heart J 1964;67:265–77. [DOI] [PubMed] [Google Scholar]
- 11. Albano A, Di Comite A, Tursi F. [Rhythmic percussion of the precordium with the closed fist as the first procedure in therapy of cardiac arrest]. Minerva Med 1967;58:2659–65. [PubMed] [Google Scholar]
- 12. Quinn TA, Kohl P, Ravens U. Cardiac mechano-electric coupling research: fifty years of progress and scientific innovation. Prog Biophys Mol Biol 2014;115:71–5. [DOI] [PubMed] [Google Scholar]
- 13. Zoll PM, Belgard AH, Weintraub MJ, Frank HA. External mechanical cardiac stimulation. N Engl J Med 1976;294:1274–5. [DOI] [PubMed] [Google Scholar]
- 14. Quinn TA. The importance of non-uniformities in mechano-electric coupling for ventricular arrhythmias. J Interv Card Electrophysiol 2014;39:25–35. [DOI] [PubMed] [Google Scholar]
- 15. Livneh A, Kimmel E, Kohut AR, Adam D. Extracorporeal acute cardiac pacing by high intensity focused ultrasound. Prog Biophys Mol Biol 2014;115:140–53. [DOI] [PubMed] [Google Scholar]
- 16. Dalecki D, Keller BB, Raeman CH, Carstensen EL. Effects of pulsed ultrasound on the frog heart: I. Thresholds for changes in cardiac rhythm and aortic pressure. Ultrasound Med Biol 1993;19:385–90. [DOI] [PubMed] [Google Scholar]
- 17. MacRobbie AG, Raeman CH, Child SZ, Dalecki D. Thresholds for premature contractions in murine hearts exposed to pulsed ultrasound. Ultrasound Med Biol 1997;23:761–5. [DOI] [PubMed] [Google Scholar]
- 18. Hersch A, Adam D. Premature cardiac contractions produced efficiently by external high-intensity focused ultrasound. Ultrasound Med Biol 2011;37:1101–10. [DOI] [PubMed] [Google Scholar]
- 19. Towe BC, Rho R. Ultrasonic cardiac pacing in the porcine model. IEEE Trans Biomed Eng 2006;53:1446–8. [DOI] [PubMed] [Google Scholar]
- 20. Rotenberg MY, Gabay H, Etzion Y, Cohen S. Feasibility of leadless cardiac pacing using injectable magnetic microparticles. Sci Rep 2016;6:24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee P, Bollensdorff C, Quinn TA, Wuskell JP, Loew LM, Kohl P. Single-sensor system for spatially resolved, continuous, and multiparametric optical mapping of cardiac tissue. Heart Rhythm 2011;8:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quinn TA, Jin H, Kohl P. Mechanically-induced premature ventricular excitation is mediated by cation non-selective stretch-activated channels and depends on the extent of local tissue deformation in isolated rabbit heart. Circulation 2011;124:A13098. [Google Scholar]
- 23. Peyronnet R, Nerbonne JM, Kohl P. Cardiac mechano-gated ion channels and arrhythmias. Circ Res 2016;118:311–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quinn TA, Granite S, Allessie MA, Antzelevitch C, Bollensdorff C, Bub G. et al. Minimum Information about a Cardiac Electrophysiology Experiment (MICEE): standardised reporting for model reproducibility, interoperability, and data sharing. Prog Biophys Mol Biol 2011;107:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper PJ, Epstein A, Macleod IA, Schaaf ST, Sheldon J, Boulin C. et al. Soft Tissue Impact Characterisation Kit (STICK) for ex situ investigation of heart rhythm responses to acute mechanical stimulation. Prog Biophys Mol Biol 2006;90:444–68. [DOI] [PubMed] [Google Scholar]
- 26. Avitall B, Levine HJ, Naimi S, Donahue RP, Pauker SG, Adam D. Local effects of electrical and mechanical stimulation on the recovery properties of the canine ventricle. Am J Cardiol 1982;50:263–70. [DOI] [PubMed] [Google Scholar]
- 27. Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation 1992;86:968–78. [DOI] [PubMed] [Google Scholar]
- 28. Dick DJ, Lab MJ. Mechanical modulation of stretch-induced premature ventricular beats: induction of mechanoelectric adaptation period. Cardiovasc Res 1998;38:181–91. [DOI] [PubMed] [Google Scholar]
- 29. Emery JL, Omens JH, McCulloch AD. Strain softening in rat left ventricular myocardium. J Biomech Eng 1997;119:6–12. [DOI] [PubMed] [Google Scholar]
- 30. Kirton RS, Taberner AJ, Young AA, Nielsen PM, Loiselle DS. Strain softening is not present during axial extensions of rat intact right ventricular trabeculae in the presence or absence of 2,3-butanedione monoxime. Am J Physiol Heart Circ Physiol 2004;286:H708–15. [DOI] [PubMed] [Google Scholar]
- 31. Hu H, Sachs F. Mechanically activated currents in chick heart cells. J Membr Biol 1996;154:205–16. [DOI] [PubMed] [Google Scholar]
- 32. Calaghan SC, White E. The role of calcium in the response of cardiac muscle to stretch. Prog Biophys Mol Biol 1999;71:59–90. [DOI] [PubMed] [Google Scholar]
- 33. Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA. et al. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 2009;104:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ward CW, Prosser BL, Lederer WJ. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal 2014;20:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belmonte S, Morad M. ‘Pressure-flow’-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol 2008;586:1379–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aston D, Capel RA, Ford KL, Christian HC, Mirams GR, Rog-Zielinska EA. et al. High resolution structural evidence suggests the sarcoplasmic reticulum forms microdomains with acidic stores (lysosomes) in the heart. Sci Rep 2016; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohl P, Cooper PJ, Holloway H. Effects of acute ventricular volume manipulation on in situ cardiomyocyte cell membrane configuration. Prog Biophys Mol Biol 2003;82:221–7. [DOI] [PubMed] [Google Scholar]
- 38. Pfeiffer ER, Wright AT, Edwards AG, Stowe JC, McNall K, Tan J. et al. Caveolae in ventricular myocytes are required for stretch-dependent conduction slowing. J Mol Cell Cardiol 2014;76:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kozera L, White E, Calaghan S. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One 2009;4:e8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gourdie RG, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov 2016;15:620–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pellman J, Zhang J, Sheikh F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: mechanisms and model systems. J Mol Cell Cardiol 2016;94:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang K, Terrar D, Gavaghan DJ, Mu UMR, Kohl P, Bollensdorff C. Living cardiac tissue slices: an organotypic pseudo two-dimensional model for cardiac biophysics research. Prog Biophys Mol Biol 2014;115:314–27. [DOI] [PubMed] [Google Scholar]
- 43. Quinn TA, Kohl P. Combining wet and dry research: experience with model development for cardiac mechano-electric structure–function studies. Cardiovasc Res 2013;97:601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.