ABSTRACT
Seedlings of three rice (Oryza sativa L.) varieties (one indica, ARC5955; and two japonica, Nipponbare and Koshihikari) with or without pre-colonization by the arbuscular mycorrhizal fungus Funneliformis mosseae were transplanted into an upland field and grown to maturity. Pre-colonization had no effect on the yield of Nipponbare or Koshihikari. However, pre-colonized ARC5955 exhibited a strong tendency toward increased yield, which was accompanied by increases in the percentage of ripened grain and the 1000-grain weight. The rice roots were also colonized by indigenous arbuscular mycorrhizal fungi in the field, but these had only limited effects on shoot biomass and grain yields. We speculate that F. mosseae may have exhibited priority effects, allowing it to dominate the rice roots. There was no significant difference in the contents of most mineral elements in the shoots of pre-colonized ARC5955 at harvest, indicating that some other factor is responsible for the observed yield increase.
KEYWORDS: Arbuscular mycorrhiza, field experiment, Funneliformis mosseae, grain yield, Oryza sativa, symbiosis
Abbreviations
- AM
arbuscular mycorrhiza or arbuscular mycorrhizal; MGR, mycorrhizal growth response
Introduction
There is currently an interest in not only elucidating the molecular mechanisms of symbiosis between rice (Oryza sativa L.) and arbuscular mycorrhizal (AM) fungi through laboratory experiments1,2 but also promoting the growth and increasing the yield of rice in the field through colonization with AM fungi. Although AM fungi are present in paddy wetlands,3,4 their colonization rate decreases with increased development of aerenchyma in the roots.5 Therefore, here we cultivated rice plants in an upland region to assess the effects of AM fungi on rice growth, like field experiments using other crops.6-8
As a first step toward identifying quantitative trait loci (QTLs), which are important for producing a positive growth response and yield increase in rice, we grew seedlings that had or had not previously been inoculated with the AM fungus Funneliformis mosseae (formerly Glomus mosseae)9,10 for 4 weeks in a glasshouse, and looked for a combination of rice varieties that showed high and low growth responses to the fungus.11 Among the NIAS Global Rice Core Collection,12 the indica variety ARC5955 showed the strongest positive growth response to colonization by F. mosseae, whereas the japonica varieties Nipponbare and Koshihikari exhibited only low response.11 Furthermore, although seedlings of ARC5955 and Nipponbare exhibited similar changes in their mineral contents following colonization, only ARC5955 experienced growth stimulation, with a particularly large increase in P content.11 Therefore, in the present study, we used pre-colonized seedlings of these three rice varieties that were grown to maturity. For field experiments, large amounts of inoculum are often needed.6,7 However, most of them can be saved through pre-colonization and transplantation of the seedlings. To our knowledge, this is the first trial to evaluate the effects of pre-colonization of AM fungus on the growth and yield of rice under natural upland conditions.
Results and discussion
In each year of the experiment, we applied less basal dressing than is standard (N, P, and K were c. 40%, 20%, and 20% of the conventional amounts, respectively) and no top dressing to the field to allow the effects of AM colonization to be clearly observed. Four 4-week-old seedlings that were at the 4–5 leaf stages and had not yet started to tiller were transplanted into each plot. The colonization percentages were between 3 and 12% for the pre-inoculated seedlings, whereas no fungal structures were observed in the control seedlings. At this point, the shoot biomass (mg) per plant of non-inoculated controls and that of pre-colonized seedlings were 110.4 ± 10.2 and 127.0 ± 6.7, respectively, for Nipponbare, 86.0 ± 4.1 and 106.7 ± 5.8, respectively, for Koshihikari, and 89.8 ± 21.1 and 141.3 ± 34.0, respectively, for ARC5955. We defined mycorrhizal growth response (MGR) by the following equation:
where DWAM and DWNM were shoot dry weights of AM and non-mycorrhizal seedlings, respectively. The MGR values (%) for Nipponbare, Koshihikari, and ARC5955 were 15.0, 24.1, and 57.3, respectively. The hill spacing was c. 50 cm × 50 cm, to minimize the cross-infection of AM fungi between the roots of different plants, and pre-colonized seedlings were transplanted into a separate bed from non-inoculated control seedlings to prevent the migration of AM fungi (Fig. 1). The mean ambient temperatures during the field experiments are shown in Fig. 2. In 2011, it became hot earlier than average, resulting in a large temperature difference between 2011 and 2012. However, according to the Ministry of Agriculture, Forestry and Fisheries of Japan (http://www.maff.go.jp/j/study/suito_sakugara/h2403/pdf/ref_data2-3.pdf), the rice yield indexes in Aichi Prefecture were the same for both years (101).
Figure 1.

Design of the field experiment conducted in 2011.
Figure 2.
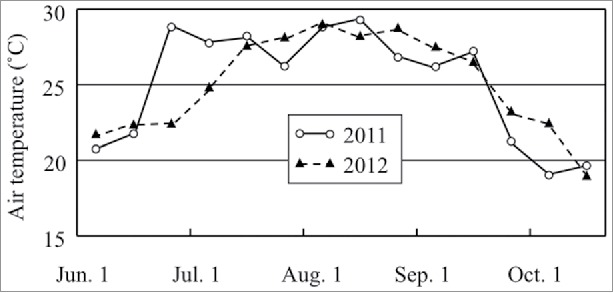
Mean ambient temperatures measured every 10 days at the experimental site at Nagoya during the cultivation periods. Solid and dashed lines represent 2011 and 2012, respectively. Source: Local Meteorological Observatory of Nagoya.
Nipponbare and Koshihikari form nonglutinous short grains, while ARC5955 forms nonglutinous long grains. Although all of these are intrinsically paddy rice varieties, we grew them in upland fields in the present experiment to remove the effect of waterlogging from the activities of the fungi. Table 1 shows the yield and yield components of the three varieties in 2011 and 2012. For Nipponbare, the pre-colonization of F. mosseae had no significant effect on any of the yield and yield components, and there was no interaction between pre-colonization and year; pre-colonization also had a variable effect on grain yield, decreasing it in 2011 and increasing it in 2012. For Koshihikari, the growth was unstable, with all of the yield and yield components showing significant year effects, possibly indicating that the upland and nutrient-poor conditions were particularly unsuitable for this variety; however, it is noteworthy that the pre-colonization of F. mosseae also had no significant effects on this variety, with a variable effect on grain yield again being observed. By contrast, ARC5955 that had been pre-colonized by the AM fungus showed a strong tendency toward increased yield in both 2011 and 2012, although this effect was not statistically significant (p = 0.070). Furthermore, the 1000-grain weight of pre-colonized ARC5955 was significantly higher than the control. It is also noteworthy that the percentage of ripened grain was also higher in pre-colonized ARC5955, and this difference was marginally significant (p = 0.054). Therefore, the yield increase that was observed in pre-colonized ARC5955 was likely due to combined increases in the percentage of ripened grain and the 1000-grain weight.
Table 1.
Yield and yield components of the three rice varieties with and without pre-inoculation by Funneliformis mosseae.
| Panicle number | Spiklet number | Total spikelet number | Percentage of ripened grain | 1000-grain weight | Grain yield | |||
|---|---|---|---|---|---|---|---|---|
| Variety |
Year |
F. mosseae |
hill−1 |
panicle−1 |
hill−1 |
(%) |
(g) |
(g hill−1) |
| Nipponbare | 2011 | − | 14.0 ± 5.3 | 85.2 ± 12.7 | 1194.0 ± 270.0 | 84.7 ± 3.1 | 23.8 ± 0.5 | 23.7 ± 4.4 |
| + | 11.9 ± 4.4 | 94.9 ± 5.3 | 1096.3 ± 372.4 | 85.7 ± 1.0 | 24.0 ± 0.7 | 22.5 ± 7.8 | ||
| 2012 | − | 9.4 ± 0.9 | 80.8 ± 4.9 | 751.3 ± 78.7 | 72.2 ± 10.1 | 21.8 ± 0.5 | 12.1 ± 2.1 | |
| + | 11.6 ± 4.7 | 88.0 ± 10.1 | 1010.0 ± 360.2 | 84.0 ± 1.9 | 22.4 ± 0.3 | 19.0 ± 6.7 | ||
| ANOVA | ||||||||
| Year | ns | ns | ns | ns (p = 0.051) | *** | ns (p = 0.051) | ||
| F. mosseae | ns | ns | ns | ns (p = 0.073) | ns | ns | ||
| Year x F. mosseae | ns | ns | ns | ns | ns | ns | ||
| Koshihikari | 2011 | − | 11.6 ± 2.2 | 84.3 ± 8.2 | 976.0 ± 120.9 | 84.4 ± 0.6 | 23.5 ± 0.5 | 19.4 ± 2.8 |
| + | 8.1 ± 1.9 | 83.7 ± 2.4 | 670.0 ± 138.1 | 84.1 ± 1.9 | 23.3 ± 0.6 | 13.1 ± 2.6 | ||
| 2012 | − | 7.0 ± 1.4 | 62.0 ± 3.5 | 369.0 ± 206.5 | 62.0 ± 3.5 | 22.0 ± 0.6 | 7.5 ± 2.2 | |
| + | 6.7 ± 0.7 | 64.8 ± 1.6 | 430.7 ± 55.3 | 64.8 ± 1.6 | 22.3 ± 1.1 | 8.2 ± 0.9 | ||
| Year | * | *** | *** | *** | * | *** | ||
| F. mosseae | ns | ns | ns | ns | ns | ns | ||
| Year x | F. mosseae | ns | ns | ns | ns | ns | * | |
| ARC5955 | 2011 | − | 24.4 ± 2.5 | 177.2 ± 23.0 | 4390.7 ± 942.1 | 89.0 ± 1.2 | 15.7 ± 0.3 | 62.3 ± 14.0 |
| + | 26.8 ± 4.7 | 188.0 ± 18.3 | 5016.7 ± 1236.3 | 93.2 ± 1.2 | 15.9 ± 0.4 | 73.7 ± 18.8 | ||
| 2012 | − | 26.8 ± 5.2 | 141.2 ± 12.6 | 3826.0 ± 814.7 | 77.2 ± 10.5 | 13.7 ± 1.1 | 41.1 ± 15.5 | |
| + | 37.1 ± 8.0 | 139.2 ± 12.5 | 5271.0 ± 1194.7 | 87.3 ± 2.8 | 15.4 ± 0.2 | 70.7 ± 18.9 | ||
| Year | ns (p = 0.079) | ** | ns | * | ** | ns | ||
| F. mosseae | ns | ns | ns | ns (p = 0.054) | * | ns (p = 0.070) | ||
| Year x | F. mosseae | ns | ns | ns | ns | ns (p = 0.078) | ns | |
Values are means ± SD (n = 3).
p < 0.05;
p < 0.01;
p < 0.001; ns, not significant.
During the cultivation period, the rice roots were also colonized by indigenous AM fungi in the field, resulting in the percentage of the root length colonized in non-inoculated controls being quite similar to that of pre-colonized plants (Table 2). Infection by indigenous fungi was also observed in 2013, although to a much lower extent than in 2011. The percentages of the root length colonized in non-inoculated controls and that of pre-colonized plants were 5.8 ± 1.2 and 16.8 ± 1.8, respectively, for Koshihikari, and 9.3 ± 1.2 and 23.5 ± 3.5, respectively, for ARC5955. Infection by indigenous AM fungi and yield increase by beneficial inoculum are in accord with the results of field experiments using alfalfa,6 maize,7 soybean and cotton.8 Jeong et al. previously performed pot experiments under upland conditions, which demonstrated that indigenous AM fungi colonize rice plants, resulting in the upregulation of AM marker genes and AM-inducible phosphate transporter genes.13 Unfortunately, however, we were unable to discriminate between pre-colonized and indigenous AM fungi under the microscope when we conducted the experiments. The differences between our results of 2011 and 2013 may have been due to the use of fallow upland that was full of weeds in 2011, as fallowed land often results in higher root colonization by AM fungi.13,14 Alternatively, it could have resulted from the use of an upland field that had freshly been converted from a paddy field in 2013. However, considering these results, we suggest that the roots of control plants in 2012 will also have been colonized by indigenous AM fungi.
Table 2.
Colonization of rice roots by arbuscular mycorrhizal (AM) fungi in 2011 at harvest. − and + indicate plants that were not inoculated and pre-inoculated with Funneliformis mosseae, respectively. Values are means ± SD (n = 3).
| Specific internal colonization |
|||||
|---|---|---|---|---|---|
| Variety | F. mosseae | Total colonization (%) | Intraradical hyphae (%) | Arbuscule (%) | Vesicle (%) |
| Nipponbare | − | 46.8 ± 7.9 | 43.1 ± 8.5 | 3.4 ± 3.1 | 0.7 ± 1.2 |
| + | 64.6 ± 0.6 | 58.0 ± 5.3 | 1.3 ± 1.1 | 6.0 ± 5.3 | |
| Koshihikari | − | 51.3 ± 3.0 | 48.0 ± 2.0 | 2.7 ± 1.1 | 0.7 ± 1.1 |
| + | 63.9 ± 1.2 | 59.3 ± 3.1 | 2.0 ± 0.0 | 3.3 ± 2.3 | |
| ARC5955 | − | 73.3 ± 2.1 | 66.7 ± 6.1 | 4.7 ± 4.6 | 2.7 ± 1.1 |
| + | 78.0 ± 6.0 | 76.7 ± 7.0 | 1.3 ± 1.1 | 0.0 ± 0.0 | |
Indigenous AM fungi appeared to have only a limited effect on grain yield, as the yield of ARC5955 was repeatedly found to increase with pre-colonization by F. mosseae. This may be because the indigenous AM fungi were not effective in improving the growth of rice plants as it is well known that the plant growth response to AM fungi depends on a combination of the plants and the fungi,15 and that there are competitive interactions between different AM fungi during root colonization.16-18 Another possibility is that an effect of each AM fungus on the grain yield might have been masked by effects of other fungi, because indigenous AM fungi consist of many species. We also speculate that F. mosseae may have exhibited priority effects, in which the AM fungus that initially colonizes the roots determines the eventual microbial population in them.19,20 Such an effect was recently demonstrated by Werner and Kiers in a glasshouse experiment with special pots, which showed that the initially inoculated AM fungus (the resident) dominates the plant roots, while another later inoculated AM fungus (the invader) fails to colonize them, likely due to host downregulation of the second fungal infection.20 Those effects can occur not only for growth-promoting fungi but also for growth-depressing fungi.21 Thus, in the present study, rice plants pre-colonized by F. mosseae, which is a beneficial fungus for ARC5955, may have blocked infection of indigenous fungi in the field. Newly established methods such as quantitative PCR targeting fungal mitochondrial genome22 and high-resolution discrimination of fungal ribosomal DNA23 may be useful to confirm the occurrence of such priority effects. Alternatively, F. mosseae will need to be tagged with a unique molecular marker that is not found in any indigenous fungi.
In 2012, we also examined the shoot biomass of the three varieties at harvest. The shoot biomass (g) per plant of non-inoculated controls and that of pre-colonized plants were 49.4 ± 18.6 and 76.0 ± 33.5, respectively, for Nipponbare, 19.3 ± 8.7 and 22.8 ± 2.0, respectively, for Koshihikari, and 126.9 ± 25.8 and 182.2 ± 34.0, respectively, for ARC5955. Interestingly, we found that pre-colonization by F. mosseae resulted in similar changes in grain yield and shoot biomass. Thus, the effects of the indigenous AM fungi again appeared to be limited, even if they infected rice roots during the cultivation period.
To understand why the shoot biomass and grain yield of pre-colonized ARC5955 increased, we measured the shoot mineral contents using ICP-AES. This showed that the Zn content was significantly lower in pre-colonized plants, while the other 10 elements remained at similar levels (Table 3). These results are in contrast with increased contents of P and N by inoculation of other crops with AM fungi.6,8 It is currently difficult to explain why a reduced Zn content would increase the grain yield, and so we cannot exclude the possibility that the different growth responses of plants may have been caused by some other factor, such as different plant hormone levels. Alternatively, the yield increase may have simply been a secondary effect of the excellent growth of ARC5955 seedlings due to F. mosseae colonization11– indeed, there is an old saying in Japan, “Nae Hansaku,” which means “If healthy seedlings are prepared, good yield of rice grains is guaranteed by half.” Therefore, a QTL analysis between ARC5955 and a japonica variety may help to clarify the mechanism of yield increase by F. mosseae, which, in turn, may be effective for improving the yield of good tasting japonica rice through the use of AM fungi in sustainable agricultural systems.
Table 3.
Mineral contents (µg/g DW) of the aboveground parts of rice at harvest. − and + indicate plants that were not inoculated and pre-inoculated with Funneliformis mosseae, respectively. Values are means ± SD (n = 3).
| Variety | F. mosseae | N | P | K | Ca | Fe | S |
|---|---|---|---|---|---|---|---|
| Nipponbare | − | 25567 ± 7315 | 1082 ± 135 | 17502 ± 2814 | 1894 ± 400 | 199 ± 26 | 1163 ± 250 |
| + | 25567 ± 8541 | 1038 ± 180 | 20609 ± 4043 | 1827 ± 261 | 192 ± 86 | 1141 ± 192 | |
| Koshihikari | − | 26933 ± 2511 | 1153 ± 249 | 15930 ± 3509 | 2003 ± 238 | 168 ± 30 | 1435 ± 438 |
| + | 24400 ± 10739 | 1130 ± 491 | 13487 ± 5042 | 1669 ± 477 | 194 ± 118 | 1424 ± 180 | |
| ARC5955 | − | 38800 ± 9111 | 1422 ± 334 | 15036 ± 2859 | 1697 ± 81 | 250 ± 70 | 1258 ± 185 |
| + | 29700 ± 3904 | 1234 ± 182 | 16494 ± 1422 | 2034 ± 257 | 301 ± 132 | 964 ± 151 |
| Variety | F. mosseae | Mg | Mn | Cu | Zn | Al |
|---|---|---|---|---|---|---|
| Nipponbare | − | 1620 ± 260 | 95 ± 8 | 10.2 ± 2.3 | 59.1 ± 19.0 | 1763 ± 99 |
| + | 1641 ± 52 | 121 ± 37 | 15.1 ± 6.9 | 36.4 ± 10.7 | 1477 ± 596 | |
| Koshihikari | − | 1673 ± 165 | 140 ± 14 | 11.9 ± 7.8 | 59.8 ± 40.9 | 943 ± 177 |
| + | 1549 ± 475 | 97 ± 28 | 6.2 ± 1.6 | 33.7 ± 7.3 | 1689 ± 917 | |
| ARC5955 | − | 1846 ± 215 | 86 ± 21 | 21.0 ± 14.6 | 47.6 ± 4.1 | 1416 ± 323 |
| + | 1855 ± 174 | 101 ± 10 | 9.4 ± 2.3 | 35.3 ± 1.1** | 1701 ± 650 |
significance at p < 0.01. A one-way ANOVA was used to analyze effects of pre-colonization.
Materials and methods
Seeds of the indica rice variety ARC5955 and the japonica rice varieties Nipponbare and Koshihikari were germinated and then transplanted individually into 40-mL cells in a plastic tray containing Kanuma soil. A soil inoculant of F. mosseae was a kind gift from Idemitsu Kosan (Tokyo, Japan). We initially found that the contaminating microorganisms in the filtrate of a soil inoculant of F. mosseae that had been passed through 38-µm mesh10 had no significant effect on plant growth. Therefore, we directly added the entire inoculant to the soil by shaking 1 L of Kanuma soil (c. 420 g) and 45 mL (30 g) of the F. mosseae inoculant in a plastic bag and then adding 40 mL of the mixture to each cell for pre-colonization. For the control, 40 mL of pure Kanuma soil was added to the cell. The seedlings were grown in a cultivation room that was maintained at 28°C under a 16 h day / 8 h night cycle at a light intensity of 204 µmol m−2 s−1 for 4 weeks. Watering was carried out as required and the soil in each cell was supplemented twice during the cultivation period with 20 mL of 0.5× modified Hoagland solution, which contained 100 µM phosphate.
The field experiments were carried out at the Higashiyama Campus of Nagoya University, Aichi Prefecture, Japan (35° 1′ N, 137° 0′ E, 61 m a.s.l.) from 2011 to 2013. In 2011, we performed the experiments in a fallow upland area that had been covered with weeds in the previous year, whereas in 2012 and 2013, we used an upland area that had been converted from flooded paddy fields. The land was plowed twice with a small tilling machine, and basal dressing (N, P, K = 2.2, 1.7, 1.5 g m−2) was applied as a 1:1 mixture (w/w) of a quick-acting compound fertilizer (Toho Co., Osaka, Japan) and the coated fertilizer Hitomaki-kun (JA Aichi, Nagoya, Japan). We then constructed beds that were 40 cm high × 1 m wide with 60-cm ditches between them, and covered these with thin black polyethylene sheets as mulch (Fig. 1). At the beginning of June, we transplanted the pre-inoculated and non-inoculated (control) seedlings into different beds. Each field was laid out in a split-plot design with three replications, whereby each plot (1 m long × 1 m wide) consisted of four plants (Fig. 1). The plants were grown until harvest at the end of October. Watering was carried out as required and no top dressing was applied during the cultivation period.
In 2011 and 2012, we harvested three and four hills, respectively, in each plot. All panicles were counted and threshed by hand, and filled and unfilled spikelets were separated and counted. After oven-drying, we determined the 1000-grain weight and calculated the grain yield for the filled unhulled grains. Unfortunately, no yield measurements could be made in 2013 because two typhoons occurred in September, severely damaging the rice plants.
In 2012, all of the stems and leaves on each of the sampled hills were also oven-dried and combined with the unhulled grains to determine shoot biomass. The combined materials were then pulverized with a small electric crusher and ground with a mortar and pestle. A portion of each ground sample was digested by heating it with HNO3, allowing the inorganic element contents to be determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES; IRIS ICAP, Nippon Jarrell Ash, Tokyo, Japan). Another portion of the ground sample was digested by heating it with NaOH and K2S2O5 and then neutralized with HCl, allowing the nitrate content to be determined spectrophotometrically, from which we calculated the nitrogen content.
In 2011 and 2013, portions of the roots were also collected at harvest, cleared with KOH, and stained with trypan blue, following which we determined the root length colonization by AM fungi (%), as described by McGonigle et al.24
We analyzed the data statistically using analysis of variance (ANOVA).
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Ms. Chiemi Sakuma and Ms. Yasuko Kato for their technical assistance and to Mr. Motoaki Kato for improvement of irrigation equipment. The first author (T.S.) was on leave from the National Agricultural and Forestry Research Institute (Lao PDR).
Funding
This work was supported in part by grants from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, PMI-0003), Japan Society for the Promotion of Science (24580020 and 16K08147) and the Global COE Program from Earth System Science to Basic and Clinical Environmental Studies.
References
- 1.Kobae Y, Hata S. Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plan Cell Physiol 2010; 51:341-53; PMID:20097910; http://dx.doi.org/ 10.1093/pcp/pcq013 [DOI] [PubMed] [Google Scholar]
- 2.Kobae Y, Gutjahr C, Paszkowski U, Kojima T, Fujiwara T, Hata S. Lipid droplets of arbuscular mycorrhizal fungi emerge in concert with arbuscule collapse. Plant Cell Physiol 2014; 55:1945-53; PMID:26979330; http://dx.doi.org/ 10.1093/pcp/pcu123 [DOI] [PubMed] [Google Scholar]
- 3.Watanarojanaporn N, Boonkerd N, Tittabutr P, Longtonglang A, Young JP, Teaumroong N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal community structure. Microbes Environ 2013; 28:316-24; PMID:23719585; http://dx.doi.org/ 10.1264/jsme2.ME13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Li T, Li Y, Björn LO, Rosendahl S, Olsson PA, Li S, Fu X. Community dynamics of arbuscular mycorrhizal fungi in high-input and intensively irrigated rice cultivation systems. Applied Environ Microbiol 2015; 81:2958-65; PMID:25681190; http://dx.doi.org/ 10.1128/AEM.03769-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallino M, Fiorilli V, Bonfante P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ 2014; 37:557-72; PMID:23927052; http://dx.doi.org/ 10.1111/pce.12177 [DOI] [PubMed] [Google Scholar]
- 6.Pellegrino E, Turrini A, Gamper HA, Cafa G, Bonari E, Young JPW, Giovannetti M. Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol 2012; 194:810-22; PMID:22380845; http://dx.doi.org/ 10.1111/j.1469-8137.2012.04090.x [DOI] [PubMed] [Google Scholar]
- 7.Berta G, Copetta A, Gamalero E, Bona E, Cesaro P, Scarafoni A, D_Agostino G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2014; 24:161-71; PMID:23995918; http://dx.doi.org/ 10.1007/s00572-013-0523-x [DOI] [PubMed] [Google Scholar]
- 8.Cely MVT, de Oliveira AG, de Freitas VF, de Luca MB, Barazetti AR, dos Santos IMO, Gionco B, Garcia GV, Prete CEC, Andrade G. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front Microbiol 2016; 7:720; PMID:27303367; http://dx.doi.org/ 10.3389/fmicb.2016.00720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krüger M, Krüger C, Walker C, Stockinger H, Schüssler A. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 2012; 193:970-84; PMID:22150759; http://dx.doi.org/ 10.1111/j.1469-8137.2011.03962.x [DOI] [PubMed] [Google Scholar]
- 10.Deguchi Y, Banba M, Shimoda Y, Chechetka SA, Suzuri R, Okusako Y, Ooki Y, Toyokura K, Suzuki A, Uchiumi T, et al.. (2007). Transcriptome profiling of Lotus japonicus roots during arbuscular mycorrhiza development and comparison with that of nodulation. DNA Res 2007; 14:117-33; PMID:17634281; http://dx.doi.org/ 10.1093/dnares/dsm014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata S, Sisaphaithong T, Suzuki S, Hanai S, Tomioka R, Kobae Y, Tanaka A, Yano K, Takenaka C. Varietal differences in growth promotion of rice (Oryza sativa) by an arbuscular mycorrhizal fungus. Abstracts of the 241st Meeting of the Crop Science Society of Japan 2016; 23**; https://www.jstage.jst.go.jp/article/jcsproc/241/0/241_23/_pdf [Google Scholar]
- 12.Kojima Y, Ebana K, Fukuoka S, Nagamine T, Kawase M. Development of an RFLP-based rice diversity research set of germplasm. Breed Sci 2005; 55:431-40; https://www.jstage.jst.go.jp/article/jsbbs/55/4/55_4_431/_pdf [Google Scholar]
- 13.Jeong K, Mattes N, Catausan S, Chin JH, Paszkowski U, Heuer S. Genetic diversity for mycorrhizal symbiosis and phosphate transporters in rice. J Integrat Plant Biol 2015; 57:969-79; PMID:26466747; http://dx.doi.org/ 10.1111/jipb.12435 [DOI] [PubMed] [Google Scholar]
- 14.Muchane MN, Jama B, Othieno C, Okalebo R, Odee D, Machua J, Jansa J. Influence of improved fallow systems and phosphorus application on arbuscular mycorrhizal fungi symbiosis in maize grown in western Kenya. Agroforest Syst 2010; 78:139-50; http://dx.doi.org/ 10.1007/s10457-009-9249-3 [DOI] [Google Scholar]
- 15.Smith SE, Read D (2008) Mycorrhizal symbiosis. (3rd ed.). San Diego: Academic Press, pp. 117-44; http://www.sciencedirect.com/science/article/pii/B9780123705266500064 [Google Scholar]
- 16.Wilson JM. Competition for infection between vesicular-arbuscular mycorrhizal fungi. New Phytol 1984; 97:427-35; http://onlinelibrary.wiley.com/doi/ 10.1111/j.1469-8137.1984.tb03608.x/epdf [DOI] [Google Scholar]
- 17.Hepper CM, Azcon-Aguilar C, Rosendahl S, Sen R. Competition between three species of Glomus used as spatially separated introduced and indigenous mycorrhizal inocula for leek (Allium porrum L.). New Phytol 1988; 110:207-15; http://onlinelibrary.wiley.com/doi/ 10.1111/j.1469-8137.1988.tb00254.x/epdf [DOI] [Google Scholar]
- 18.Pearson JN, Abbott LK, Jasper DA. Phosphorus, soluble carbohydrates and the competition between two arbuscular mycorrhizal fungi colonizing subterranean clover. New Phytol 1994; 127:101-6; http://dx.doi.org/ 10.1111/j.1469-8137.1994.tb04263.x [DOI] [PubMed] [Google Scholar]
- 19.Verbruggen E, van der Heijden MGA, Rillig MC, Kiers ET. Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success. New Phytol 2013; 197:1104-9; PMID:23495389; http://dx.doi.org/ 10.1111/j.1469-8137.2012.04348.x [DOI] [PubMed] [Google Scholar]
- 20.Werner GDA, Kiers ET. Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytol 2015; 205:1515-24; PMID:25298030; http://dx.doi.org/ 10.1111/nph.13092 [DOI] [PubMed] [Google Scholar]
- 21.Janoušková M, Seddas P, Mrnka L, van Tuinen D, Dvořáčková A, Tollot M, Tollot M, Gianinazzi-Pearson V, Vosátka M, Gollotte A. Development and activity of Glomusintraradices as affectedby co-existence with Glomus claroideum in one root system. Mycorrhiza 2009; 19:393-402; PMID:19377892; http://dx.doi.org/ 10.1007/s00572-009-0243-4 [DOI] [PubMed] [Google Scholar]
- 22.Badri A, Stefani FO, Lachance G, Roy-Arcand L, Beaudet D, Vialle A, Hijri M. Molecular diagnostic toolkit for Rhizophagus irregularis isolate DAOM-197198 using quantitative PCR assay targeting the mitochondrial genome. Mycorrhiza 2016; 26:721-33; PMID:27220880; http://dx.doi.org/ 10.1007/s00572-016-0708-1 [DOI] [PubMed] [Google Scholar]
- 23.Schlaeppi K, Bender SF, Mascher F, Russo G, Patrignani A, Camenzind T, Hempel S, Rillig MC, van der Heijden MG. High-resolution community profiling of arbuscular mycorrhizal fungi. New Phytol 2016; 212:780-91; PMID:27381250; http://dx.doi.org/ 10.1111/nph.14070 [DOI] [PubMed] [Google Scholar]
- 24.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 1990; 115:495-501; http://dx.doi.org/ 10.1111/j.1469-8137.1990.tb00476.x [DOI] [PubMed] [Google Scholar]
- **In Japanese with English title. [Google Scholar]


