Figure 6. Afatinib radiosensitizes HNSCC tumors in vivo.
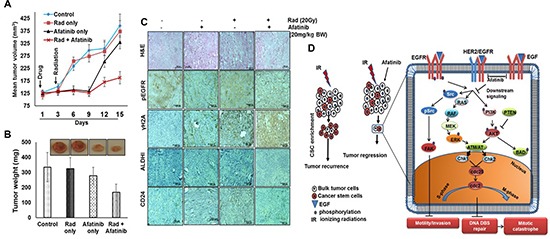
(A) SCC1 cells were subcutaneously implanted on contralateral flanks in athymic nude mice and randomized into group 1 (8 animals each) with afatinib treatment and radiation on the right tumors, and afatinib only on the left side tumors; group 2 (8 animals each) with vehicle gavages and radiation on the right tumors. Tumor volume and animal weights were measured every 3 days starting from the day of drug administration. All the mice were sacrificed on the 15th day after afatinib treatment and body weight and tumor weight measured. The graphs show a significant decrease in tumor volume (A) and tumor weights (B) in afatinib + IR treated animals compared to control, afatinib only, or IR-treated mice. (C) Excised tumors were analyzed for pEGFR (Tyr-1068), pγH2AX (Ser-139), CD24, and ALDH1 expression using immunohistochemical analysis (×20 magnification). (D) Schematic diagram illustrating the potential molecular mechanism of afatinib mediated radio-sensitization of HNSCC. Treatment of ionizing radiation (IR) kills the bulk of tumor cells but enriches cancer stem cells shown as red (left side) that leads to tumor recurrence. However, pre-treatment of afatinib radio-sensitizes tumors and inhibits both CSCs and the bulk of tumor cells, and results in significant tumor shrinkage. The molecular mechanism revealed that afatinib significantly inhibited the phosphorylation of EGFR, HER2, and HER3 coupled with inhibition of downstream signaling molecules, including pAkt (Ser-473) and pERK1/2 (Thr202/Tyr204). Afatinib pre-treatment abrogated IR-induced activation of DNA DSB repair by inhibiting pAkt (Ser-473) and pERK1/2 (Thr202/Tyr204), pATM (Ser-1981), pChk2 (Thr-68), pATR (Ser-428) and pBRCA1 (Ser-1524). In addition, afatinib inhibited IR-induced SP and NSP population and downregulated expression of cancer stem cell markers CD44 and Oct3/4.
