Abstract
PURPOSE
We characterized factors related to non-definitive management of high-risk prostate cancer patients, and assessed impact from race, insurance status, and facility-level volume of technologically-advanced prostate cancer treatments (i.e. intensity-modulated radiation therapy, robotic-assisted laparoscopic radical prostatectomy) on this outcome.
METHODS
We identified men with high-risk localized prostate cancer (based on D’Amico criteria) in the National Cancer Data Base (2010–2012). Primary outcome was non-definitive management (i.e., delayed/no treatment with prostatectomy/radiation therapy or androgen deprivation therapy monotherapy). Treating facilities were classified by quartiles of proportions of patients treated with advanced technology. Multivariable regression estimated odds of primary outcome based on race, insurance status, and facility-level technology use, and evaluated for interactions between these covariates.
RESULTS
Among 60,300 patients, 9265 (15.4%) received non-definitive management. This was more common among non-White men (p<0.001), Medicaid/uninsured patients (p<0.001), and those managed at facilities in the lowest quartile of technology use (25.1% vs 11.0% highest, p<0.001). Though non-definitive management was common among non-White men with Medicaid/no insurance treated at low-technology centers (43% vs 10% White, private/Medicare, high-tech facility; adjusted OR 7.18, p<0.001), this was less likely if this group was managed at a high-tech hospital (22% vs 43% low-tech, p<0.001).
CONCLUSIONS
Technology-use at a facility correlates with high-quality prostate cancer care, and is associated with diminished disparities based on insurance status and patient race. More research is required to characterize other facility-level factors explaining these findings.
Keywords: disparities, technology, prostate cancer, insurance coverage, African-Americans
1. INTRODUCTION
Delivery of equitable high-quality cancer care remains a challenge since the Institute of Medicare reported on disparities in health care in 2002.1 These issues persist for non-White men considering prostate cancer treatment. For men with high-risk prostate cancer (HRCaP), guidelines recommend therapy with radiation (RT) in conjunction with androgen deprivation (ADT) or radical prostatectomy (RP) with pelvic lymphadenectomy.2 This is based on the finding that many patients with HRCaP managed with observation will die of prostate cancer within 10 years from diagnosis.3 Despite this, definitive treatment for men with HRCaP is underutilized4 and disparities exist based on race and insurance status.5, 6
One major underpinning to these disparities is impaired access to quality cancer care, as medically-underserved populations do not differ in response to prostate cancer treatment.7 Health systems permitting more equal access (e.g., Veterans Health Administration (VHA)) have not demonstrated racial disparities in cancer care seen elsewhere.8 One barrier to equitable care could be access to novel technology for prostate cancer treatment, such as robotic-assisted laparoscopic radical prostatectomy (RALRP) and intensity-modulated radiation therapy (IMRT). Adoption of these technologies is associated with increased treatment volume9,10, and considerable attention has been given to implications of this adoption on overtreatment of men with indolent prostate cancer.10 However, it is unknown whether this technology adoption is associated with more appropriate treatment of HRCaP patients. Additionally, it is unclear whether medically-underserved patients—based on race and insurance status—have impaired access to these technologically-advanced treatment sites and this access would be associated with diminished disparities in HRCaP treatment.
To that end, we evaluated relationships between (a) patient race and insurance coverage and (b) hospital-level volume of advanced prostate cancer treatment (i.e., RALRP/IMRT) with receipt of non-definitive management (NDM) of men with HRCaP. We hypothesized that non-White and Medicaid/uninsured patients would be less likely to receive prompt treatment of high-risk tumors, and access to high-technology cancer centers would be associated with more equitable HRCaP treatment. If confirmed, our findings would have implications for prostate cancer patients seeking high-quality treatment, and policymakers eager to identify targets to diminish disparities in delivery of appropriate cancer care.
2. METHODS
2.1 Data Source
We used the National Cancer Data Base (NCDB) that captures ~70% of newly-diagnosed cancers in the United States diagnosed or treated at hospitals recognized by the American College of Surgeons’ Commission on Cancer.11
2.2 Cohort identification (Supplemental Figure 1)
We identified men ≥ 30 years old diagnosed with T1-3N0M0 CaP from 2010–2012 (n=311,693). We excluded patients who were (a) diagnosed at autopsy, (b) received all treatment at a non-reporting facility, (c) previously diagnosed with another cancer, (d) missing race or rural/urban status, or (e) followed for ≤ 6 months after diagnosis. To ensure statistical reliability of facility-level measures, we excluded patients at facilities diagnosing ≤ 30 patients. We limited this cohort to HRCaP patients based on D’Amico criteria (i.e., Gleason score ≥ 8, clinical T3 stage, PSA ≥ 20.0 ng/mL).12
2.3 Outcome of interest
Our primary outcome of interest was NDM of HRCaP defined as (a) no primary treatment with RP/RT, (b) RP/RT ≥ 6 months after diagnosis, and (c) androgen deprivation (ADT) monotherapy. Our cutoff of 6 months for delayed treatment is based on a recent systematic review for the appropriate window between diagnosis and treatment of HRCaP.13 Patients with an unknown date of treatment were considered as having received definitive treatment, based on finding that <10% of patients in our cohort who underwent RP/RT were treated ≥ 6 months after diagnosis.
2.4 Exposures of interest and other covariates
We dichotomized race and insurance coverage as “White” versus “non-White” and “Private/Medicare” versus “Medicaid/uninsured,” respectively. We considered IMRT or RALRP as technologically-advanced (versus 3D-conformal RT or open retropubic RP). To calculate facility-level use, we utilized the entire cohort diagnosed with localized CaP from 2010–2012, regardless of risk. Calculated proportions used the number of localized CaP cases treated with RP/RT per facility per year as the denominator and the number of cases treated with IMRT/RALRP per facility per year as the numerator. We stratified facilities into quartiles of advanced treatment based on these proportions.
We also considered potentially confounding variables, including patient/geographic characteristics (age, distance to facility, rural/urban status, comorbidity, region), area socioeconomic factors (% no high school degree, median income), and facility factors (i.e., academic/community hospital). Census-tract based socioeconomic factors were based on data from 2008–2012. Rural-urban status was defined using rural-urban commuting area codes assigned using 2013 data14, and categorized as metropolitan vs suburban/rural. Distance to facility is defined by the NCDB as the great circle distance from patient residence and diagnosing facility.
2.5 Statistical Analysis
We performed parametric and non-parametric testing to generate summary statistics and evaluate associations between NDM, exposures, and potential confounders. We constructed our initial multivariable logistic regression model with NDM as our outcome, and included covariates determined a priori, including four interaction terms related to our exposures of interest (i.e., race*insurance, race*technology, insurance*technology, race*insurance*technology). We used generalized linear mixed-effects model with Huber-White sandwich estimators to account for biased estimates due to clustering at the facility level.15, 16 Our models were calibrated by including our hypothesis-based selected covariates and removing non-significant interaction terms using backwards stepwise selection with a significance threshold of p=0.05. After removing non-significant interactions, our next model included race, insurance status, facility-level technology use, and adjusted for age, comorbidity, year of diagnosis, facility type, geographic region, PSA, Gleason grade, rural/urban residence, distance to facility, median income and % high school degree in Census tract, and two significant interaction terms (race*technology, race*insurance*technology). The final model replaced race, insurance, and facility-level technology covariates with a 16-level variable that allowed estimation of odds ratios between all combinations of race, insurance, and technology (with White, Private/Medicare, high-tech facility as reference), while adjusting for other covariates and clustering at facility-level. All analyses were performed with SAS version 9.3 (Cary, NC). All tests were two-sided and p<0.05 were considered statistically significant. Our institutional review board deemed this study as exempt from their oversight due to de-identified data.
3. RESULTS
For our cohort, the mean age was 66±9 years, with 83% younger than 75 years of age. The majority were White (82%), had Medicare/private insurance coverage (94%), and lived in a metropolitan region (82%) (Supplemental Table 1). Most of the high-risk patients had either zero (82%) or one (15%) comorbid condition. The majority of patients had T1–T2 tumors (90%), Gleason score 8–10 (58%), and PSA < 20 (67%). Median PSA was 9.4 (IQR 5.4–26.2).
Among 60,330 HRCaP patients, 9265 (15.4%) underwent NDM. Most NDM was either lack of receipt of RP/RT (n=6490, 68.8%) or ADT monotherapy (n=1881, 19.9%). Multivariable regression estimated that NDM was significantly more common among older patients (adjusted OR (aOR) 2.25 per 10 years of age, 95%CI 2.17–2.33) and among patients with 2 or more comorbid conditions (aOR 1.63, 95%CI 1.43–1.87) (Table 1). Patients with higher-risk Gleason grade 8–10 tumors were less likely to undergo NDM (15.8% vs 17.8% GS 3+3=6, aOR 0.61, 95%CI 0.56–0.66).
Table 1.
Non-definitive management of high-risk prostate cancer based on patient, facility, and tumor factors.
| NDM | Multivariable Regression* | |
|---|---|---|
|
| ||
| n (row %) | OR (95% CI) | |
|
| ||
| Race | ||
| White | 6977 (14.2) | Reference |
| Non-White | 2288 (20.7) | 1.51 (1.36 – 1.68) |
| Insurance Coverage | ||
| Medicare/private | 8138 (14.5) | Reference |
| Medicaid/uninsured | 959 (27.7) | 1.97 (1.77 – 2.20) |
| Facility-level Technology Use | ||
| Low-tech (Q1) | 1985 (25.1) | 2.16 (1.73 – 2.69) |
| Q2 | 2545 (18.0) | 1.46 (1.20 – 1.79) |
| Q3 | 2518 (13.8) | 1.08 (0.89 – 1.33) |
| High-tech (Q4) | 2217 (11.1) | Reference |
| Facility-level Case Volume | ||
| Low-volume (Q1) | 517 (25.0) | 1.53 (1.21 – 1.95) |
| Q2 | 1550 (19.9) | 1.23 (1.05 – 1.45) |
| Q3 | 2508 (16.5) | 1.11 (0.96 – 1.27) |
| High-volume (Q4) | 4691 (13.3) | Reference |
| Year of diagnosis | ||
| 2010 | 3147 (14.8) | Reference |
| 2011 | 3082 (15.1) | 1.03 (0.96 – 1.09) |
| 2012 | 3036 (16.3) | 1.08 (1.01 – 1.16) |
| Age (per 10 years) (mean (SD)) | 70.9 (10.2) | 2.25 (2.17 – 2.33) |
| Charlson Comorbidity Score | ||
| 0 | 7319 (14.8) | Reference |
| 1 | 1496 (16.2) | 1.05 (0.98 – 1.13) |
| 2+ | 450 (25.9) | 1.63 (1.43 – 1.87) |
| Rural-urban residence | ||
| Metropolitan | 7629 (15.4) | Reference |
| Suburban/Rural | 1636 (15.2) | 0.91 (0.83 – 1.01) |
| Distance to Facility (miles) | ||
| 0–25 | 7238 (16.1) | Reference |
| 26–100 | 1583 (13.6) | 0.99 (0.91 – 1.08) |
| > 100 | 373 (11.2) | 0.83 (0.72 – 0.96) |
| Median income of census tract | ||
| < $38,000 | 2105 (19.2) | 1.10 (0.97 – 1.24) |
| $38,000–35,999 | 2249 (16.0) | 1.05 (0.95 – 1.17) |
| $48,000–62,999 | 2394 (14.7) | 1.02 (0.93 – 1.11) |
| $63,000+ | 2504 (13.2) | Reference |
| % no high school degree in census tract | ||
| > 20.0 | 2028 (20.1) | 1.18 (1.05 – 1.34) |
| 13.0–20.0 | 2530 (16.6) | 1.10 (1.00 – 1.22) |
| 7.0–12.9 | 2694 (13.8) | 1.00 (0.92 – 1.09) |
| < 7.0 | 2003 (13.0) | Reference |
| Facility Type | ||
| Community | 5705 (15.0) | Reference |
| Academic/Research | 3553 (16.2) | 1.33 (1.16 – 1.53) |
| Gleason Score | ||
| 3+3=6 | 1569 (17.8) | Reference |
| 3+4=7 or 4+3=7 | 2183 (14.0) | 0.53 (0.49 – 0.58) |
| 8–10 | 5196 (15.8) | 0.61 (0.56 – 0.66) |
| PSA (ng/mL) | ||
| 0–10 | 2940 (10.1) | 0.46 (0.43 – 0.49) |
| 10–20 | 1218 (14.2) | 0.57 (0.52 – 0.62) |
| 20+ | 4101 (22.1) | Reference |
Based on multivariable regression model which included all listed covariates, region (9-level categorical), two interaction terms (race*technology, race*insurance*technology) with adjustment for clustering at the facility-level and excluded patients with missing data (final n = 52,839)
Bolded text signifies p<0.05.
Abbreviations: NDM – non-definitive management; OR – odds ratio; Q – quartile; SD – standard deviation; PSA – prostate-specific antigen.
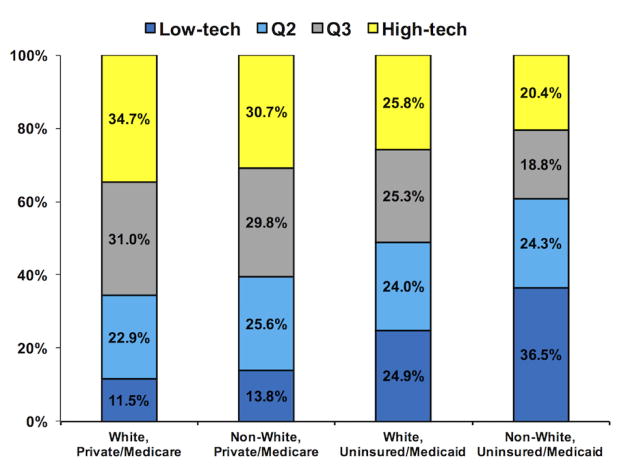
Well-insured patients were more likely to be managed at high-tech facilities, compared to men with Medicaid/no insurance coverage (p<0.001) (Figure 1). In particular, non-White men with Medicaid or no insurance were considerably less likely to use these high-tech facilities (20.4% vs 34.7% White, private/Medicare, p<0.001). Although there were no racial disparities in access to low-tech facilities for well-insured men (13.8% non-White vs 11.5% White, p>0.05), non-White men who were uninsured or with Medicaid were much more likely to be managed at low-tech centers compared to White men with similar insurance coverage (36.5% non-White vs 24.9% White, p<0.001).
Figure 1. Variation in utilization of low- and high-tech facilities based on patient race and insurance status.
This figure displays distribution of utilization of facilities based on patient race and insurance status. Non-White men with Medicaid or no insurance were much more likely to be treated at low-tech facilities (36.5% vs 20.4% high-tech, p<0.001). On the contrary, White men with Medicare or private insurance coverage were much more likely to be treated at high-tech facilities (34.7% vs 11.1%, p<0.001).
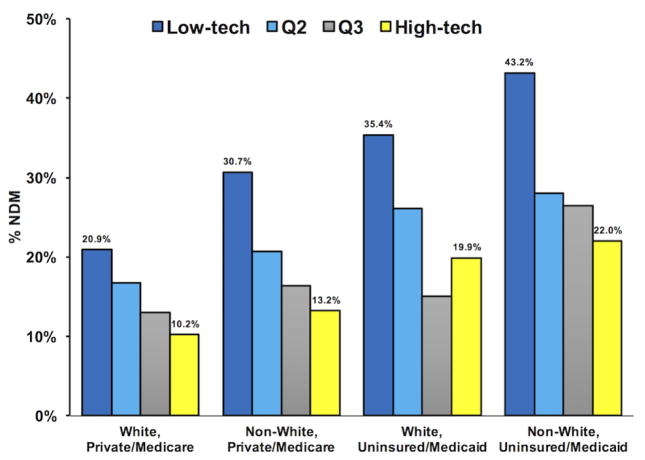
Within each cluster of race and insurance combination (e.g., White, Private/Medicare coverage), there was an inverse relationship between NDM and facility-level technology use, with NDM consistently less common as facility-level technology use increased (all p<0.001) (Figure 2). Additionally, we also noted a consistent relationship between patient race, insurance status, and NDM within each quartile of facility-level technology use. Having private or Medicare coverage clearly was associated with less frequent receipt of NDM, compared to Medicaid/no coverage, across all racial groups and facility quartiles (all p<0.001). That is, use of NDM was significantly much more likely to be received by men who were non-White and had Medicaid/no insurance in both low- (43.2% vs 20.9% White with private insurance/Medicare, p<0.001) and high-tech facilities (22.0% vs 10.2% White with private insurance/Medicare, p<0.001).
Figure 2. Receipt of non-definitive management by high-risk prostate cancer patients based on patient race, insurance coverage, and facility-level technology use.
This figure displays the proportions of patients that received NDM of their high-risk tumors (y-axis), based on combinations of patient race and insurance coverage (x-axis). Receipt of NDM was consistently less common at high-tech facilities, across all race/insurance groups (all p<0.001). Furthermore, over 43% of non-White men with Medicaid/no insurance who were treated in low-tech facilities underwent NDM (vs 21% White, well-insured, low-tech facility, p<0.001).
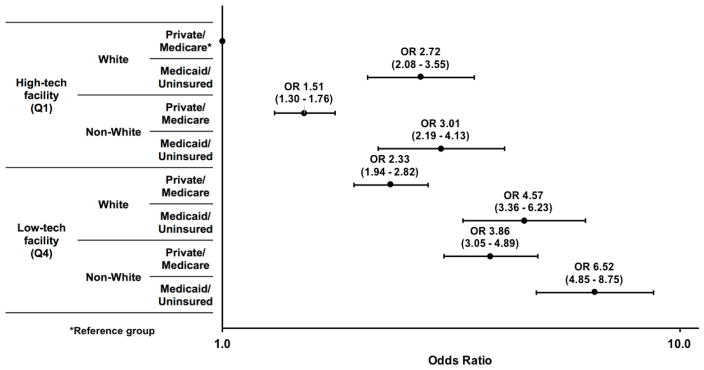
The estimated odds ratios from our final multivariable model are shown in Figure 3. As expected, non-White HRCaP patients who had Medicaid/no insurance and were managed at low-tech facilities were the most likely to receive NDM; this outcome was over seven times as likely in this group compared to White, well-insured men managed at high-tech hospitals (aOR 6.52, 95% CI 4.85 – 8.75). However, non-White men with Medicaid/no insurance were only three times as likely to receive NDM diagnosis at a high-tech facility, demonstrating diminished disparities in this setting (aOR 3.01, 95% CI 2.19 – 4.13). Having private or Medicare coverage diminished this disparity even further, as non-White, well-insured men treated in high-tech facilities were only 1.5 times as likely to receive NDM, compared to White well-insured men in the same setting (aOR 1.51, 95% CI 1.30 – 1.76)
Figure 3. Estimated odds ratios of non-definitive management from multivariable model evaluating interactions between patient race, insurance status, and facility-level technology.
This figure shows the estimates from our final multivariable model, which included a 16-level variable that allowed estimation of odds ratios (shown on logarithmic scale on x-axis) between all combinations of race, insurance, and technology (with White, Private/Medicare coverage, high-tech facility as reference) while adjusted for age (continuous), comorbidity, year of diagnosis, facility type (academic vs community), geographic region, PSA (categorical), Gleason grade, rural/urban residence, distance to facility (categorical), and median income and % high school degree in Census tract (quartiles). Non-White HRCaP patients with Medicaid/no insurance who were managed at low-tech facilities were over seven times as likely to receive NDM, compared to well-insured White men managed at high-tech facilities (OR 7.18, 95% CI 5.37 – 9.61). However, these odds decreased significantly with treatment at a high-tech facility (OR 3.02, 2.20 – 4.15) and more so with addition of private/Medicare coverage (OR 1.51, 95% CI 1.30 – 1.76). *reference group
4. DISCUSSION
Our study has three principal findings. First, among all men with HRCaP identified by the NCDB, non-White men with HRCaP were significantly less likely to receive definitive treatment compared to White men. Second, high-quality insurance coverage was associated with a decrease in racial disparity in prostate cancer treatment across all practice settings. Finally, a diagnosis of HRCaP at a high-tech facility was also associated with an additional shift toward racial equanimity in prompt treatment of HRCaP.
The pervasive undertreatment of men with HRCaP has been well-established, which some ascribe to the dogma that older men with a life-expectancy less than 10 years do not require aggressive treatment of their disease, regardless of tumor risk.17 However, guidelines—albeit consensus-based—recommend that all patients with HRCaP should pursue treatment with either RT combined with ADT or RP with pelvic lymphadenectomy when possible, and primary ADT should only be reserved for patients for whom those therapies would be contraindicated (e.g., prior pelvic radiation).2 These guidelines are supported by population-based observational data demonstrating the overall survival benefit of RT plus ADT (vs ADT alone) for older men who have locally-advanced HRCaP.18 Despite this, nearly two thirds of high-risk patients over 75 years of age diagnosed within a population-based collaborative were treated with primary ADT from 2010–2013.4 In addition, nearly 30% of high-risk patients overall underwent primary ADT or were not treated (which was twice as common as what we report herein). No matter what the practice setting, there are clear gaps between what is supported by guidelines and clinical evidence and what is actually being delivered to patients in need.
In particular, patients of color are at increased risk of not receiving appropriate treatment of their prostate cancer, even when matched for patient and tumor characteristics.19–21 Even if they eventually undergo prostatectomy, Black patients have greater delays to treatment and less frequent receipt of lymphadenectomy compared to White men.19 Among our cohort, non-White HRCaP patients were nearly 50% more likely to receive NDM. However, to say that these differences are solely due to patient race may improperly discount other factors such as insurance coverage and lack of access to high-quality care. It is well-established that non-White patients are more likely to have inferior health insurance coverage compared to their White counterparts.22 Furthermore, a lack of adequate insurance explains up to one-quarter of gaps in health care access seen by persons of color.22 A recent population-based analysis demonstrated that insurance coverage was associated with a greater likelihood of treatment among Black patients with HRCaP, compared to those with no coverage.6 We also found that well-insured non-White patients were considerably more likely to receive prompt treatment of their high-risk tumors, compared to those with either Medicaid or no insurance.
However, having insurance is not enough by itself. For instance, cancer patients covered by Medicaid present with more advanced disease and are less likely to receive cancer-directed therapy, compared to those with private/Medicare coverage.23,24 Furthermore, it matters which hospitals patients can access. In California, disadvantaged prostate cancer patients experienced different treatment patterns between county and private hospitals.25 Though undertreatment of HRCaP persists, high-tech cancer centers may be bright spots of guideline-concordant care for this population. Perhaps most importantly, we also found that management of HRCaP patients at these hospitals demonstrated diminished racial disparities among the patients able to gain access. Gaining this access, however, was limited for non-White men and those with Medicaid/no insurance, as they were less likely to be managed at facilities commonly utilizing IMRT and RALRP. Ensuring equitable access can help eliminate racial disparities in cancer care, as in the VHA and other integrated health systems.8, 26
Our findings must be placed in the context of limitations of our analysis. First, the NCDB captures patients at CoC-accredited hospitals, which introduces selection bias, as underserved patients are less likely to receive care at those sites.27 However, the NCDB represents ~70% of all cancer cases diagnosed in the United States. Second, without assessing functional status and severity of comorbid diseases, we cannot evaluate the “appropriateness” of therapy for these patients. Nevertheless, the majority of the cohort is younger than 75 with minimal comorbidity and would likely have >10 years of life expectancy and benefit from treatment of their high-risk disease. Third, though insurance coverage and access to high-tech centers was associated with decreases in racial disparities, we must consider barriers related to patient income and educational level not captured in this study. Rural residence and increased distance to treating facilities are both established barriers to care for prostate28, and bladder cancer patients.29 However, we adjusted for socioeconomic factors that held marginal statistical significance in our final models. Furthermore, increasing distance to facilities was inversely associated with receipt of NDM among HRCaP patients. Finally, as with any retrospective analysis of an observational dataset, we cannot capture patient preferences, which certainly play an important component on the decision to pursue aggressive treatment of HRCaP.
Despite those limitations, insurance coverage and access to technology were associated with decreases in racial disparities in definitive treatment for HRCaP patients. The Affordable Care Act made inroads in expanding coverage for non-White patients, and decreasing coverage disparities based on race.30 Policy-wise, increasing access for non-White HRCaP patients to high-tech centers could abrogate disparities in cancer care. However, the marginal benefits associated with robotic-assisted surgery and IMRT are still being actively evaluated. For instance, though observational data have demonstrated improved outcomes with RALRP compared to open surgery31, recent randomized trial data say otherwise.32 Thus, the adoption of technology itself may simply be a proxy for facility-level characteristics linked with high-quality cancer care. Considerable work is still required to understand what other factors are linked with this facility-level use of technology that are permitting more prompt treatment of HRCaP patients (e.g., presence of a multidisciplinary clinic, fellowship-trained physicians, etc.).
In conclusion, private or Medicare insurance coverage and facility-level use of technologically advanced treatments were both associated with considerable decreases in racial disparities of HRCaP care.
Supplementary Material
HIGHLIGHTS.
One in eight men with high risk prostate cancer received non-definitive management
Lack of insurance/non-White race were associated with higher risk of non-definitive management
Access to high-tech centers associated with less disparity in high-risk prostate cancer treatment
Acknowledgments
Funding:This work was supported by funding from the Winship Cancer Center Prostate Cancer Pilot Grant (CPF). Research was also supported by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI (grant number P30CA138292). The authors have no conflicts of interest to report.
Footnotes
Disclaimers:
The content is solely the responsibility of the authors and does not represent the official views of the NIH. The data are derived from a de-identified NCDB file. The American College of Surgeons and Commission on Cancer have not verified and are not responsible for analytic or statistical methodology employed, or the conclusions drawn from these data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: National Academies of Sciences; 2002. [PubMed] [Google Scholar]
- 2.Mohler JL, Armstrong AJ, Bahnson RR. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 3.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 5.Wang EH, Yu JB, Abouassally R, et al. Disparities in treatment of patients with high-risk prostate cancer: results from a population-based cohort. Urology. 2016;95:88. doi: 10.1016/j.urology.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Mahal BA, Ziehr DR, Aizer AA, et al. Getting back to equal: The influence of insurance status on racial disparities in the treatment of African-American men with high-risk prostate cancer. Urol Oncol. 2014;32:1285. doi: 10.1016/j.urolonc.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Canc Inst. 2002;94:334. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 8.Daskivich TJ, Kwan L, Dash A, et al. Racial parity in tumor burden, treatment choice and survival outcomes in men with prostate cancer in the VA healthcare system. Prostate Cancer Prostatic Dis. 2015;18:104. doi: 10.1038/pcan.2014.51. [DOI] [PubMed] [Google Scholar]
- 9.Barbash GI, Glied SA. New technology and health care costs — the case of robot-assisted surgery. N Engl J Med. 2010;363:701. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 13.van den Bergh RCN, Albertsen PC, Bangma CH, et al. Timing of curative treatment for prostate cancer: a systematic review. Eur Urol. 2013;64:204. doi: 10.1016/j.eururo.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washington Wyoming Alaska Montana Idaho Rural Health Research Center. WWAMI Rural Health Research Center: Rural-Urban Communting Area Codes (RUCAs) http://depts.washington.edu/uwruca/
- 15.White H. Maximum Likelihood Estimation of Misspecified Models. Econometrica. 1982;50:1. [Google Scholar]
- 16.Huber PJ. The proceedings of the fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, CA: University of California Press; 1967. [Google Scholar]
- 17.Shumway DA, Hamstra DA. Ageism in the undertreatment of high-risk prostate cancer: how long will clinical practice patterns resist the weight of evidence? J Clin Oncol. 2015;33:676. doi: 10.1200/JCO.2014.59.4093. [DOI] [PubMed] [Google Scholar]
- 18.Bekelman JE, Mitra N, Handorf EA, et al. Effectiveness of androgen-deprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015;33:716. doi: 10.1200/JCO.2014.57.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85. doi: 10.1001/jamaoncol.2015.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moses KA, Paciorek AT, Penson DF, et al. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2010;28:1069. doi: 10.1200/JCO.2009.26.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chornokur G, Dalton K, Borysova ME, et al. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71:985. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lillie-Blanton M, Hoffman C. The role of health insurance coverage in reducing racial/ethnic disparities in health care. Health Aff. 2005;24:398. doi: 10.1377/hlthaff.24.2.398. [DOI] [PubMed] [Google Scholar]
- 23.Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32:3118. doi: 10.1200/JCO.2014.55.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SP, Boorjian SA, Shah ND, et al. Disparities in access to hospitals with robotic surgery for patients with prostate cancer undergoing radical prostatectomy. J Urol. 2013;189:514. doi: 10.1016/j.juro.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Parsons JK, Kwan L, Connor SE, et al. Prostate cancer treatment for economically disadvantaged men: a comparison of county hospitals and private providers. Cancer. 2010;116:1378. doi: 10.1002/cncr.24856. [DOI] [PubMed] [Google Scholar]
- 26.Rhoads KF, Patel MI, Ma Y, et al. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol. 2015;33:854. doi: 10.1200/JCO.2014.56.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of Commission on Cancer-approved and non-approved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 28.Muralidhar V, Rose BS, Chen Y-W, et al. Association between travel distance and choice of treatment for prostate cancer: does geography reduce patient choice? Intl J Radiat Oncol Biol Phys. 2016 doi: 10.1016/j.ijrobp.2016.05.022. in press. [DOI] [PubMed] [Google Scholar]
- 29.Gore JL, Litwin MS, Lai J, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Vargas-Bustamante A, Mortensen K, et al. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care. 2016;54:140. doi: 10.1097/MLR.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu JC, O’Malley P, Chugtai B, et al. Comparative effectiveness of cancer control and survival after robot-assisted versus open radical prostatectomy. J Urol. 2016 doi: 10.1016/j.juro.2016.09.115. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.