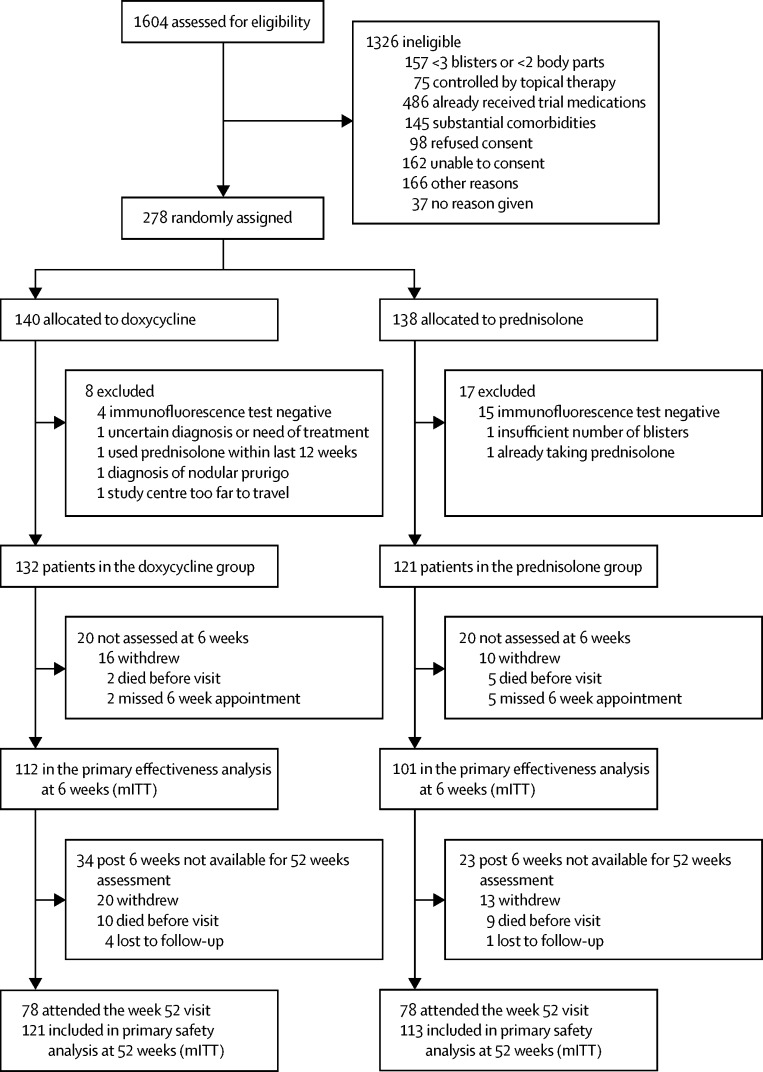
Figure 1.
Trial profile for the primary outcome population
Those patients excluded from analysis at week 6 due to missing their week 6 assessment are included in the denominator at week 52, as they had the possibility of attending a visit after week 6 and therefore were not considered lost to follow-up at week 6. Patients who did not attend their week 52 visit are called lost to follow-up at week 52. The primary safety analysis (mITT) was based on 121 and 113 participants allocated to initial doxycycline and prednisolone, respectively, who had at least one return visit. Multiple imputation was used for missed visits as specified in the protocol. mITT=modified intention to treat.