Abstract
An excess of calcium (Ca2+) influx into mitochondria during mitochondrial re-energization is one of the causes of myocardial cell death during ischemic/reperfusion injury. This overload of Ca2+ triggers the mitochondrial permeability transition pore (mPTP) opening which leads to programmed cell death. During the ischemic/reperfusion stage, the activated Ca2+/calmodulin-dependent protein kinase II (CaMKII) enzyme is responsible for Ca2+ influx. To reduce CaMKII-related cell death, sub-micron particles composed of poly(lactic-co-glycolic acid) (PLGA), loaded with a CaMKII inhibitor peptide were fabricated. The CaMKII inhibitor peptide-loaded (CIP) particles were coated with a mitochondria targeting moiety, triphenylphosphonium cation (TPP), which allowed the particles to accumulate and release the peptide inside mitochondria to inhibit CaMKII activity. The fluorescently labeled TPP-CIP were taken up by mitochondria and successfully reduced ROS caused by Isoprenaline (ISO) in a differentiated rat cardiomyocyte-like cell line. When cells were treated with TPP-CIP prior ISO exposure, they maintained mitochondrial membrane potential. The TPP-CIP protected cells from ISO-induced ROS production and decreased mitochondrial membrane potential. Thus, TPP-CIP have the potential to be used in protection against ischemia/reperfusion injury.
Keywords: PLGA, CaMKII, mitochondria targeting
1. Introduction
Myocardial infarction (MI) or heart attack, is a commonly observed symptom of coronary heart disease which is a leading cause of death and disability worldwide1. According to the American Heart Association, one American will have a MI approximately every 42 seconds2. Typically, reducing acute myocardial ischemic injury and limiting the infarct size using thrombolytic therapy to gain myocardial reperfusion is an effective therapeutic intervention3. However, the injury caused during ischemia/reperfusion remains. One of the causes of myocardial cell death during ischemia/reperfusion injury in MI patients is an excess of calcium (Ca2+) influx into mitochondria during mitochondrial membrane potential (ΔΨ) restoration4. This overload of Ca2+ leads to mitochondria permeability transition pore (mPTP) opening and increased levels of reactive oxygen species (ROS), which eventually leads to myocardial cell death. The multifunctional calcium/calmodulin (Ca2+/CaM) dependent protein kinase II (CaMKII) enzyme is rapidly activated during the ischemia/reperfusion stage and is responsible for mitochondrial Ca2+ influx5, 6. Inhibiting CaMKII in the mitochondria reduces cell death from ischemia/reperfusion injury in vivo5. A potent CaMKII inhibitor (CaMKIIN) protein is thought to reduce Ca2+ flux into mitochondria, thereby preventing mPTP opening7. Chang et al. have identified a 21 amino acid sequence (CaMKIIN peptide, Supplementary Figure 1A) derived from CaMKIIN protein at the amino acid sequence 43 – 63, has exhibited potent CaMKII inhibitory activity8, 9.
To achieve a therapeutic effect, CaMKIIN peptide needs to be able to enter the cells and reach mitochondria. All studies performed so far have focused on modifying the protein/peptide itself to increase cellular uptake or target the mitochondria. Examples include mtCaMKIIN (which contains a mitochondrial localization sequence)5, palmitoyl-CaMKIIN (which contains a membrane localization motif)5 and TatCN21 (a membrane permeable CaMKIIN peptide)10. Besides delivery of the peptide to cardiomyocyte mitochondria, avoiding physiological degradation of the peptide must be considered. Submicron sized particles made from poly(lactic-co-glycolic acid) (PLGA, structure can be found in Supplementary Figure 1B), an FDA-approved biodegradable polymer, can be loaded with various molecules such as peptides/proteins and protect them from degradation by proteases11, 12. The PLGA polymer can be conjugated with various targeting moieties13, 14 and therefore could be used to increase cellular uptake and deliver the CaMKIIN peptide to mitochondria. The targeting moiety chosen for this current study was the triphenylphosphonium cation (TPP, Supplementary Figure 1D). In 1984, TPP was reported to have an uptake and binding ability to the matrix face and cytosolic face of the mitochondria inner membrane15. Since then, TPP has been a popular choice for mitochondria targeting strategies. Moreover, TPP has been reported to promote the rate and amount of cellular internalization of conjugated nanomaterials16. There are various TPP-conjugated drug molecules and bioactive compounds such as, vitamin E (MitoVit E)17, a ubiquinone derivative (MitoQ)18 and doxorubicin19. There are also liposomes formulated with TPP in the lipid layers to target mitochondria20. TPP can be conjugated to PLGA-block-poly(ethylene glycol) (PEG) polymer and blended with either non-targeted PLGA-block-PEG-OH or PLGA-COOH to prepare solid PLGA particles using a nanoprecipitation method21. These particles can be loaded with lipophilic drugs such as curcumin and 2, 4-dinitrophenol, and were reported to be taken up into the mitochondria of human cervical cancer (HeLa) cells.21.
The well characterized cell line, H9c2, derived from rat cardiac tissue22, was used in this study. This cell line has been used extensively as a model for ischemia/reperfusion injury22–26 due to its high sensitivity to ischemia/reperfusion injury in terms of cell viability and mitochondrial respiration24. Moreover, H9c2 cells have been used in a number of studies involving CaMKII27–30. In these studies, the chronic exposure to all-trans retinoic acid was used to induce H9c2 cells to differentiate from cardiomyoblast-like to cardiomyocyte-like cells as has been previously described31.
Instead of modifying the peptide structure to achieve an increase in both cellular uptake and mitochondrial targeting, in this report, we describe a new drug delivery system for transporting CaMKIIN peptide to mitochondria using functionalized PLGA particles. To the best of our knowledge, this is the first time that a CaMKII inhibitor peptide has been incorporated into a drug delivery system, specifically, into surface-modified PLGA particles. These submicron sized PLGA particles loaded with CaMKIIN peptide were prepared using a modified double emulsion solvent diffusion/evaporation method. The particle surface was conjugated with TPP, using carbodiimide crosslinker chemistry. The ability of the particles to inhibit CaMKII activity was tested using differentiated H9c2 cells that were treated with isoprenaline.
2. Materials and methods
2.1 Fabrication of PLGA Particles
CaMKIIN peptide-loaded particles (CIP) were made using two types of polymer: PLGA (PLGA 50:50 lactic acid: glycolic acid, ester endcap, MW: 24,000 – 38,000 Da; Resomer® RG503, Boehringer Ingelheim Pharma Gmbh & Co., Ridgefield, CT, Supplementary Figure 1B) and PLGA-NH2 (50:50 lactic acid: glycolic acid, diamine endcap, MW: 10,000 – 20,000 Da; PolySciTech Division, Akina, Inc., West Lafayette, IN, Supplementary Figure 1C). The fluorescently-labeled CaMKIIN peptide (H-KRP PKL GQI GRA KRV VIE DDR K(HF488)-NH2) was custom produced (Anaspec, Inc. (EGT group), Fremont, CA) with a purity ≥ 90% according to HPLC analysis by the company. CaMKIIN peptide-loaded particles (CIP) were prepared using a double emulsion solvent diffusion/evaporation method with some modifications32–34. Briefly, 138 μL containing 625 μg of CaMKIIN peptide in 1% polyvinyl alcohol (PVA, Mowiol® 8–88, Mw ∼67,000, Sigma-Aldrich®, St. Louis, MO) was sonicated into a solution of 50 mg of PLGA and 50 mg of PLGA-NH2 dissolved in 150 μL of dimethyl sulfoxide (DMSO) and 2.5 mL of ethyl acetate. Sonication using a Sonic Dismembrator Ultrasonic processor (Fisher Scientific, Pittsburgh, PA) was conducted at 40% amplitude for 45 seconds to create the primary emulsion. To create the secondary emulsion, the primary emulsion was sonicated in 9 mL of 2.5% PVA in 1× PBS containing 1 mL of ethyl acetate at 40% amplitude for 60 seconds. The emulsion was then poured into 2.5% PVA in 1× PBS (51 mL), and stirred in a fume hood for 30 minutes for the particles to solidify. Separation/pelleting of unwanted particles (> 300 nm) was performed by differential centrifugation at 4500 × g for 5 minutes (Eppendorf centrifuge 5804 R, Eppendorf, Hauppauge, NY). The desired particles in the supernatant were then collected by centrifugation at 10,000 × g for 30 minutes (Thermo Scientific Sorvall™ Legend™ XTR Centrifuge, 230VAC, Cole-Parmer, Vernon Hills, IL) and were washed twice with sterile water, and then frozen and lyophilized using a FreeZone 4.5-L Benchtop Freeze Dry System (Labconco Corporation, Kansas City, MO). This particle preparation method is illustrated in Figure 1A.
Figure 1.
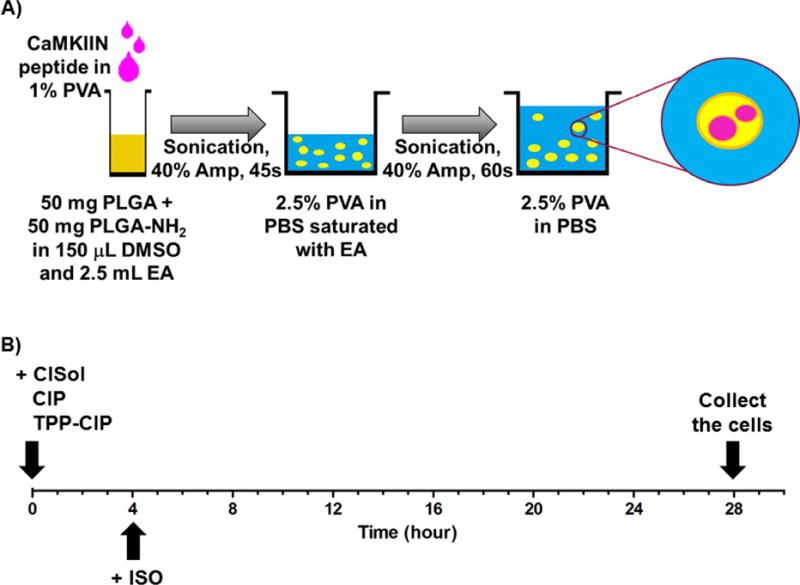
(A) Fabrication of CaMKIIN-loaded particles (CIP): schematic of the protocol for loading CaMKIIN peptides into PLGA particles (see method for further details). PLGA, poly(lactic-co-glycolic-acid); PLGA-NH2, amine endcapped poly(lactic-co-glycolic-acid); PVA, polyvinyl alcohol; EA, ethyl acetate; Amp, amplitude.
(B) Timeline of in vitro experiments with differentiated H9c2 cells: cells were treated with different formulations of CaMKIIN peptide at t = 0, incubated with ISO at t = 4 and collected at t = 28. CaMKIIN peptide solution (CISol); CaMKIIN loaded particles (CIP); TPP conjugated CaMKIIN loaded particles (TPP-CIP); Isoprenaline (ISO).
Particle surfaces were functionalized with (4-carboxybutyl) triphenylphosphonium bromide (TPP, Sigma-Aldrich) using carbodiimide crosslinker chemistry (ThermoFisher Scientific, Waltham, MA) during the fabrication process. In this reaction, a TPP-derivative containing a carboxylic acid (-COOH) functional group was used along with a mixture of ester end capped PLGA and amine end capped PLGA (PLGA-NH2) in particle fabrication. Thus, the carbodiimide compound was used to activate carboxylic acids for subsequent primary amine conjugation through the formation of amide bonds. While the particles were stirred in the fume hood, 4 mL of (triphenyl phosphate) - (1-ethyl-3-3(3-dimethylaminopropyl) carbodiimide HCl) -(N-hydroxysucciniide) (TPP-EDC-NHS solution) in 0.1 M 2- (N-morpholino) ethanesulfonic acid (MES) buffer solution was added dropwise. Particles were reacted with this solution for 2 hours before being collected, washed and lyophilized. Excess molar ratios of TPP, EDC and NHS were used. For 1 mg PLGA and PLGA-NH2 polymer, 1 mg, 4 mg and 6 mg, of TPP, EDC and NHS were used, respectively.
2.2 Quantification of CaMKIIN peptide loading in CIP
The loading capacity was quantified using a method described by Joshi et al35. Since CaMKIIN peptide was fluorescently labeled, the peptide loading in lyophilized particles could be quantified using a SpectraMax® plus 384 Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA) (excitation/emission: 500/530). A known amount of lyophilized CIP were degraded using 0.3 N NaOH. After all particles were degraded, the solution was neutralized with 1 N HCl to approximately pH 7 then the fluorescence intensity was measured and compared to the intensity of standard CaMKIIN peptide solutions in 1× PBS. The linear range for the CaMKIIN peptide is between 0.05 – 6.25 μg/mL.
2.3 Morphology, size and zeta potential of particles
The surface morphology of particles was assessed using Scanning Electron Microscopy (SEM: Hitachi S-4800, Hitachi High-Technologies, Ontario, Canada). The lyophilized particles prepared using the method mentioned above were resuspended in deionized water. A drop of this particle suspension (approximately 10 μL) was placed onto a silicon wafer mounted on a SEM stub using double stick carbon tape and air dried. The silicon wafer was then coated with gold-palladium using a sputter coater (K550 sputter coater, Emitech Ltd., Kent, England). The images were collected at 1.5 kV accelerating voltage. The size and zeta potential from each batch of particles were measured using a Zetasizer Nano ZS (Malvern, Worcestershire, UK).
2.4 Confirmation of TPP conjugation on the particle surface using fluorescamine
Fluorescamine interacts with primary amines to yield highly fluorescent derivatives36. In this study, fluorescamine was used to quantify the availability of primary amines on the surface of particles prepared using the mixture of PLGA and PLGA-NH2 (as an indirect measurement of TPP conjugation). Three types of particles were tested, blank (without CaMKIIN peptide) particles made from PLGA and PLGA-NH2 (PLGA:PLGA-NH2, 50:50), TPP conjugated-blank particles made from PLGA and PLGA-NH2 (TPP-PLGA:PLGA-NH2, 50:50) and blank particles made purely from ester-end capped PLGA (PLGA:PLGA-NH2, 100:0).
Lyophilized particles were resuspended in 1 mL PBS. Then, 50 μL of fluorescamine dissolved in acetone (9 mg/ml) was added to the particles. The reaction was allowed to continue for 5 minutes. The particles were then washed three times with PBS. The fluorescence intensity of the particle surface was determined using flow cytometry (LSR II flow cytometer, Becton Dickinson, New Jersey, NJ).
2.5 Cell lines and cell culture
Rat cardiomyoblast-derived cells (H9c2, ATCC® CRL-1446™) were maintained in DMEM medium (Gibco®, Life technologies, Grand Island, NY). All of the media were supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 2 mM Glutamax (Gibco®) and 1% penicillin/streptomycin (Gibco®). Cells were incubated at 37°C and 5% CO2. Subculturing was performed before cells reached 80% confluency in order to prevent the loss of differentiation potential37.
According to Comelli et al., H9c2 cells can differentiate into cardiomyocyte-like cells using a low percentage (1%) of FBS in combination with retinoic acid31. H9c2 cells were seeded at a concentration of 4 × 105 cells/well in 150-mm dishes using DMEM supplemented with 1% FBS, 10 nM all-trans retinoic acid, 2 mM Glutamax (Gibco®) and 1% penicillin/streptomycin (Gibco®). Media was replenished every two days. Differentiated H9c2 cells were used after 7 days of differentiation. Cells were maintained in the differentiation media throughout all experiments.
2.6 Flow cytometry analysis of Mitotracker® Red stained cells
Seven days after the differentiation process, H9c2 cells were gently rinsed with pre-warmed PBS, trypsinized with 0.25% trypsin-EDTA and collected using centrifugation at 230 × g for 5 mins. Cells were then incubated with 200 nM Mitotracker® Red (CMSRos, Molecular Probes, Life technologies, Eugene, OR) for 15 mins in an incubator (37°C, 5% CO2) to stain the mitochondria. Cells were collected by centrifugation and resuspended in pre-warmed PBS without any fixatives. The fluorescent signal from Mitotracker® Red was quantified immediately using flow cytometry (FACScan: Becton Dickinson Immunocytometry Systems, San Jose, CA). Undifferentiated H9c2 cells incubated in DMEM media supplemented with 10% FBS were used as a control group.
2.7 Measurement of intracellular reactive oxygen species (ROS) production by dihydroethidium (DHE) staining
After H9c2 cells were differentiated, the CaMKIIN peptide in either solution or particulate form was added into each 150-mm dish at a concentration of 100 nM, 4 hours prior to adding 125 μM of isoprenaline (ISO). Cells were incubated with particles and ISO for 28 and 24 hours, respectively. The treatment groups (CISol (CaMKIIN peptide in soluble form), CIP (CaMKIIN peptide loaded particles), TPP-CIP (TPP functionalized CaMKIIN peptide loaded particles) and ISO were maintained in the media until the end of the experiment. After the exposure, media was removed. Cells were gently rinsed with pre-warmed PBS to remove excess particles (see treatment timeline: Figure 1B). Cells were trypsinized with 0.25% trypsin-EDTA and collected by centrifugation at 230 × g for 5 mins. The cells were washed with pre-warmed PBS containing 5 mM sodium pyruvate and incubated at 37°C with dihydroethidium (DHE, 10 μM in DMSO) in PBS containing 5 mM sodium pyruvate. After 40 mins incubation, the cells were analyzed using flow cytometry (FACScan). The relative mean fluorescence intensity (MFI) of 20,000 cells was recorded. All groups were normalized to the untreated control group. Antimycin A or AntA (an electron transport chain blocker, 10 μM in DMSO) was used as a positive control and subsequently increased the DHE oxidation levels by 2.6-fold higher than the control group (data not shown).
2.8 Quantification of particle uptake by differentiated H9c2 cells
The excitation and emission wavelengths of oxidized product from DHE are 535 and 610 nm, respectively38. The CaMKIIN peptide was tagged with a fluorophore (HF488, HiLyte Flour™ Dye, Anaspec. Inc,) possessing excitation and emission wavelengths of 500 and 530 nm, respectively. ROS production in H9c2 cells and the fluorescent signal from the CaMKIIN peptide were measured simultaneously using flow cytometry. The relative mean fluorescence intensities (MFI) of 20,000 cells were recorded. All groups were normalized to the untreated control group.
2.9 Measurement of mitochondrial membrane potential by Tetramethylrhodamine, methyl ester, Perchlorate (TMRM) staining
Differentiated H9c2 cells were seeded at 1×105 cells per dish one day prior to the experiment. Cells were treated in the same manner as described in section 2.7. After the exposure, the medium was removed. Cells were trypsinized and resuspended in 250 μL of media which contained 25 μM tetramethylrhodamine, methyl ester, perchlorate (TMRM) in Hanks’ balanced salt solution (HBSS). Cells were exposed to TMRM containing media in the dark for 30 mins at 37°C. After the incubation was completed, the old media was replaced with DMEM media containing 1% FBS, retinoic acid and sodium pyruvate. Cell images were obtained using an epi-fluorescence, inverted Olympus IX-81.
The fluorescent intensity from H9c2 mitochondria in each group was obtained using the raw intensity data (F1) / background intensity data (F0) which is the area in the image with no cells.
2.10 Statistical Analysis
Data are expressed as mean ± SEM (Scatter dot plot). Statistical significance was determined using an unpaired T-test or One-Way ANOVA with Bonferroni’s multiple comparison tests. All statistical tests were performed using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla CA, www.graphpad.com). A p-value less than 0.05 was considered significant.
3. Results and Discussion
3.1 Particle characterization
CIP were fabricated using a water in oil in water double emulsion solvent diffusion/evaporation method as previously described with some modifications32, 33. Ethyl acetate was used as the organic phase with PVA as a stabilizer. This method was developed in order to load water soluble drugs into nanometer-sized particles. The hydrodynamic diameters for CIP (PLGA/PLGA-NH2) and TPP-CIP (TPP-PLGA/PLGA-NH2) were approximately 210.4 ± 3.92 nm (n=5) and 276.5 ± 41.51 nm (n=3), respectively, with a narrow size distribution (Supplementary Figure 2). All particles had a negative overall surface charge. The drug loading of CIP (PLGA/PLGA/NH2) and TPP-CIP (TPP-PLGA/PLGA-NH2) were approximately 0.47 ± 0.04 μg/mg lyophilized particles (n=3) and 0.38 ± 0.06 μg/mg lyophilized particles (n=3), respectively. Entrapment efficiency of CIP was approximately 10.15%. Production yield of NPs (% yield) was approximately 57.16 ± 9.05 (n=5). The surface morphology of the particles was studied using SEM and they were shown to have smooth surfaces, were spherical in shape and were uniform in size (Figure 2). A release study was performed in order to determine the drug release profile from CIP (Supplementary Figure 4). The results indicated that more than 50% of CaMKIIN peptide was released from the particles within the first hour and more than 90% within 13 days.
Figure 2. Representative scanning electron micrograph of particles made from PLGA and PLGA-NH2 with TPP functionalization on the particle surface.
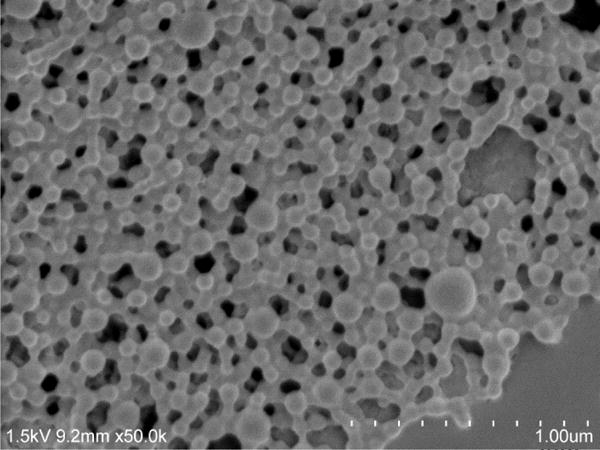
Scale bars represent 1.00 μm.
3.2 TPP was successfully conjugated onto the particle surface
To create mitochondria-targeting particles, TPP was conjugated onto the particle surface using carbodiimide crosslinker chemistry as described in the methods. Two assays were performed to confirm TPP-particle conjugation: 1) zeta potential measurements and 2) detection of residual primary amines with fluorescamine. Since TPP is a lipophilic cation its successful conjugation onto the particle surface results in an increase in zeta potential39. As shown in Figure 3, TPP-PLGA:PLGA-NH2, 50:50 particles demonstrated significantly higher zeta potential when compared to unmodified particles (PLGA:PLGA-NH2, 50:50). These results suggest that the TPP group was present on the particle surface of the TPP-PLGA:PLGA-NH2, 50:50 particles. The second method that was used to confirm TPP conjugation involved treating the particles with the amine-reactive dye, fluorescamine. In theory, if the particle surface contains free primary amines, it will react with fluorescamine to yield a highly fluorescent product. However, if the particles surface was occupied with TPP group, there would be lower number of free amines to react with fluorescamine. Thus, TPP-conjugated particles should yield a lower fluorescence intensity compared to unconjugated particles. Schematic images representing the different types of particles made in this experiment are shown in Figure 4A. In Figure 4B, TPP-PLGA:PLGA-NH2, 50:50 particles that were incubated with fluorescamine showed significantly lower fluorescence intensity (p-value < 0.05) compared to PLGA:PLGA-NH2, 50:50 particles that were incubated with fluorescamine. The control group (PLGA particles without primary amines, PLGA:PLGA-NH2, 100:0) showed no significant fluorescence intensity. The results obtained from this experiment were consistent with zeta potential measurements (Figure 3) and suggested that TPP was successfully conjugated onto the surface of the particles.
Figure 3. Zeta potential of particles before and after TPP conjugation.
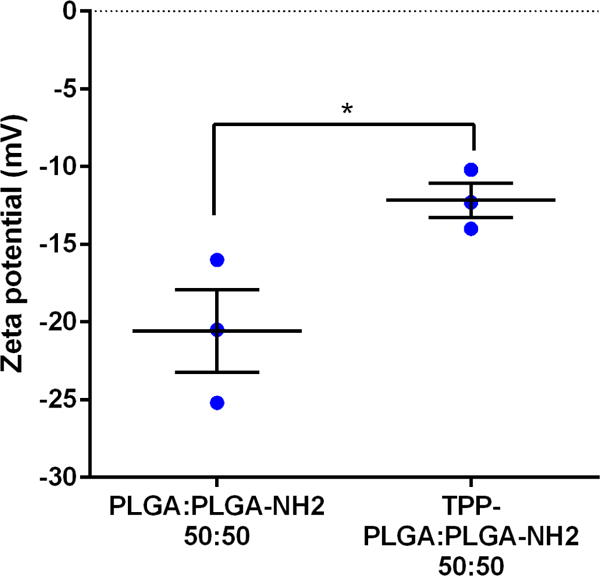
PLGA: PLGA-NH2, 50:50represents particles prepared using a mixture of PLGA and PLGA-NH2 at the ratio of 50:50. TPP- PLGA: PLGA-NH2, 50:50 represents particles made from mixture of PLGA and PLGA-NH2 at the ratio of 50:50 with TPP conjugation. Data are expressed as scatter plots (n = 3). Unpaired two-tailed t-test was conducted to determine significant differences between PLGA: PLGA-NH2, 50:50 and TPP-PLGA: PLGA-NH2, 50:50.
*p < 0.05.
Figure 4. TPP conjugation to PLGA:PLGA-NH2 particles.
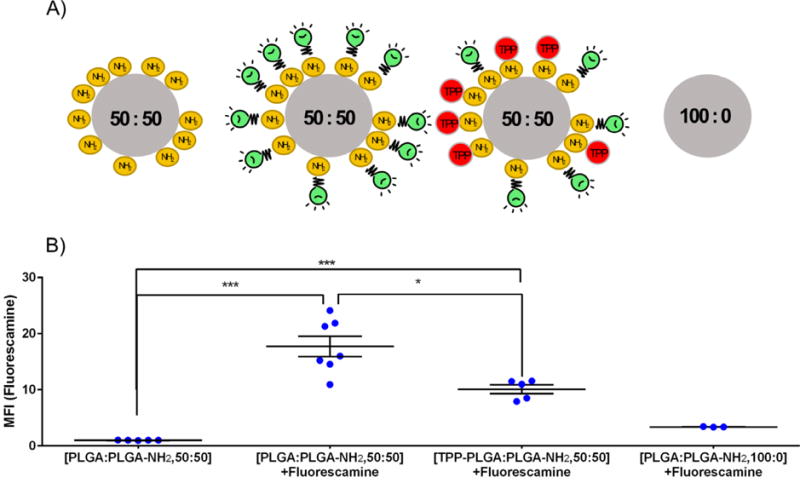
(A) Schematic of particles with and without TPP conjugation (PLGA:PLGA-NH2 and TPP-PLGA:PLGA-NH2) and incubated with fluorescamine. (B) Mean fluorescence intensity (MFI) obtained from particles treated with/without fluorescamine as indicated. PLGA:PLGA-NH2, 50:50 = particles made from a mixture of PLGA and PLGA-NH2 at the ratio of 50:50; TPP-PLGA:PLGA-NH2, 50:50 = TPP-conjugated particles made from a mixture of PLGA and PLGA-NH2 at the ratio of 50:50; PLGA:PLGA-NH2, 100:0 = particles made purely from PLGA with no amine functionalization. Green light bulbs represent fluorescamine. Data are expressed as scatter plots (n = 3 – 7). One-way analysis of variance with Bonferroni’s multiple comparisons test was performed. ***p < 0.001, *p < 0.05.
3.3 Differentiated H9c2 cells have mitochondrial content higher than undifferentiated H9c2 cells
Undifferentiated H9c2 cells are used as an alternative to primary cardiomyocytes as they are recognized as being representative of the cardiomyoblast lineage22, 40. These cells have the ability to differentiate into cardiomyocyte-like cells when they are exposed to all-trans retinoic acid in reduced-serum media. After differentiation, H9c2 cells express a more cardiac-like phenotype such as having a greater mitochondrial mass31, as well as having an increased expression of proteins involved in Ca2+ handling and mitochondrial metabolism41. Such characteristics are of importance in these studies since the particles being tested were designed to target the mitochondria of cardiomyocytes. Moreover, there have been reports suggesting that differentiated H9c2 cells were more susceptible to cytotoxic substances such as isoprenaline42 and doxorubicin37 than undifferentiated cells. Since this study is focused on the protective ability of the particles against isoprenaline-induced toxicity, differentiated cells which are more sensitive to toxic substances, would generate more distinct results than undifferentiated cells.
Flow cytometric analysis of Mitotracker® Red fluorescence was used as a semiquantitative indicator of mitochondrial mass. After 7 days of differentiation in the presence of all-trans retinoic acid and 1% FBS, H9c2 cells had a relative mean fluorescence intensity (MFI) 2.2-fold higher than untreated cells (Figure 5). It has been previously established that treating H9c2 cells with all-trans retinoic acid for 7 days results in an increase in mitochondrial content which is considered one of the markers for the differentiation-associated changes in mitochondrial biogenesis31. The aforementioned flow cytometry data was verified using confocal microscopy which showed an increase in mitochondrial mass after Mitotracker® Red staining (Supplementary Figure 5). Taken together, these results confirmed that significant differentiation of H9c2 cells had been achieved.
Figure 5. Relative mitochondrial mass as determined using Mitotracker® Red.
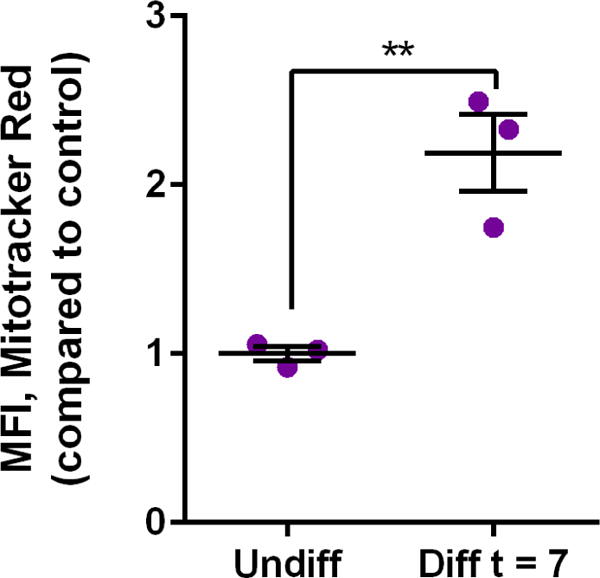
Relative mean fluorescence intensity (MFI) due to Mitotracker® Red comparing two groups of cells: H9c2 cells that were exposed to the differentiation agent, all-trans-retinoic acid, for 7 days (Diff t = 7) and H9c2 cells not treated with all-trans retinoic acid (Undiff). Data are expressed as scatter plots (n = 3). Unpaired two-tailed t-test was performed. **p < 0.01.
3.4 TPP-CIP were most efficiently taken up by differentiated H9c2 cells compared to other particle formulations
After differentiated H9c2 cells were incubated with various CaMKIIN peptide formulations and ISO, cells were collected and analyzed for particle uptake using flow cytometry, exploiting the fluorescent signal from the CaMKIIN peptide itself. Cells that were treated with TPP-CIP had the highest fluorescent signal amongst all groups tested which was 14.7-fold higher than the untreated cells (control group, Figure 6A). Cells that were incubated with CIP and CISol had similar levels of fluorescence with 4.1- and 3.5-fold higher than untreated cells, respectively. Thus, using TPP conjugation to the surface of PLGA particles significantly increased cellular uptake by differentiated H9c2 cells.
Figure 6. Enhanced uptake of, and reduced pro-oxidants levels due to, TPP-CIP in H9c2 cells.
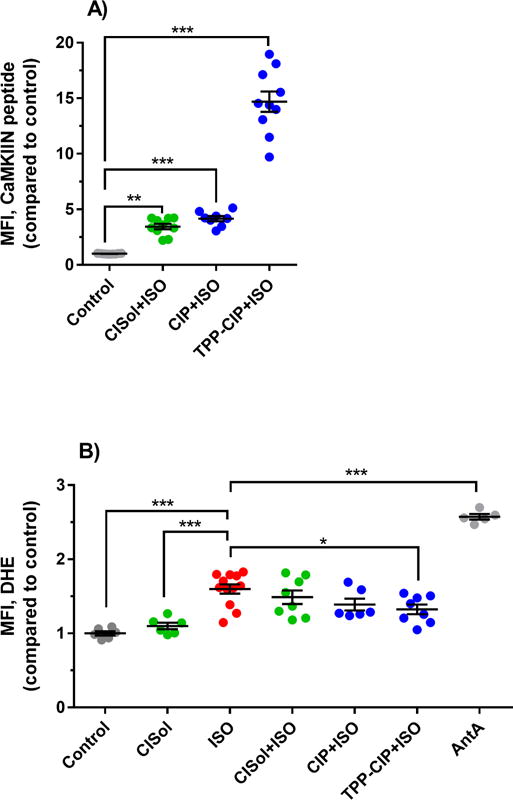
(A) Mean fluorescence intensity (MFI) from fluorescently-tagged CaMKIIN peptide obtained from cells that were incubated with CaMKIIN solution (CISol), CaMKIIN loaded particles (CIP) and TPP-conjugated CaMKIIN loaded particles (TPP-CIP) and treated with isoprenaline (ISO). Data are expressed scatter plots, (n = 8 – 12). One-way analysis of variance with Bonferroni’s multiple comparisons test compared to the control was performed. ***p < 0.001, **p < 0.01. (B) Mean fluorescence intensity (MFI) due to the presence of intracellular pro-oxidants as detected by dihydroethidium oxidation (DHE) obtained from cells that were incubated with CaMKIIN solution (CISol), CaMKIIN loaded particles (CIP) and TPP-conjugated CaMKIIN loaded particles (TPP-CIP) and treated with isoprenaline (ISO). Antimycin A (AntA) was used as a positive control. AntA increased the MFI by 2.6-fold when compared to the control group. Data are expressed as scatter plots, (n = 5 – 12). One-way analysis of variance with Bonferroni’s multiple comparisons test compared to the ISO was performed. ***p < 0.001, **p < 0.01, *p < 0.05.
3.5 TPP-CIP reduced intracellular ROS induced by ISO
In the same set of experiments, differentiated H9c2 cells were also analyzed for intracellular ROS (pro-oxidants such as superoxide) induced by ISO (Figure 6B). Without the CaMKIIN peptide, cells that were treated with 125 μM ISO for 24 hours had 1.6-fold higher fluorescent signal, generated by the oxidized DHE product, than the control group. When cells were pre-treated with CaMKIIN peptide in either soluble form (CISol) or particulate forms (CIP, TPP-CIP) 4 hours prior to treatment with ISO, the intracellular ROS decreased. Among the different CaMKIIN peptide formulations, TPP-CIP had the lowest fluorescent signal (1.3-fold higher than the control group) and was therefore the most effective at mitigating ROS levels (p-value < 0.05 when compared to ISO-treated cells).
3.6 TPP-CIP maintained mitochondrial membrane potential in differentiated H9c2 cells after ISO treatment
Differentiated H9c2 cells were treated with ISO with and without CIP or TPP-CIP pretreatment. Mitochondrial membrane potential was measured using TMRM. Cells treated with ISO alone or cells pretreated with CIP then ISO showed a decrease in membrane potential compared to that of cells pre-treated with TPP-CIP (Figure 7). This suggests that cells pretreated with TPP-CIP had more polarized mitochondria compared to other groups.
Figure 7. Effect of pretreatment with CIP at maintaining the mitochondria membrane potential in ISO-treated H9c2 cells.
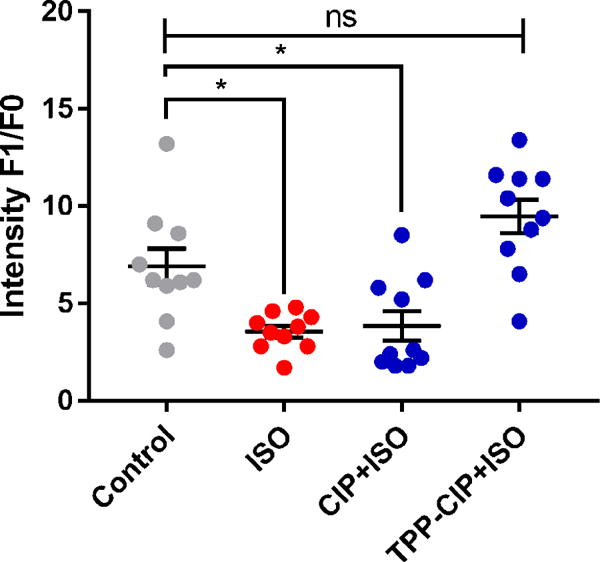
TMRM intensity (F1/F0) obtained from epi-fluorescence images of H9c2 cells that were stained with TMRM dye after incubation with either CAMKIIN loaded particles (CIP) or TPP-conjugated CaMKIIN loaded particles (TPP-CIP) then treated with isoprenaline (ISO). F1 represents the raw intensity data. F0 represents the background intensity data which were obtained from the areas of the images that contained no cells. Control group is untreated cells. Data are expressed as scatter plots, (n = 10). One-way analysis of variance with Bonferroni’s multiple comparisons test compared to the control group was performed. *p < 0.05. ns, not statistically significant.
In the studies presented here, a CaMKIIN peptide was successfully entrapped inside PLGA particles. These PLGA particles comprised a mixture of PLGA (ester endcapped) with PLGA-NH2 (amine endcapped) and, in order to impart mitochondrial targeting capabilities to these particles, a test group was conjugated to TPP by linking the carboxylate group on TPP to the amine group on PLGA-NH2. TPP was conjugated onto the surface of PLGA particles using carbodiimide crosslinker chemistry. Confirmation of successful TPP conjugation to the surface of the particles was achieved through zeta potential measurements of the particles and assessing amine availability on the particle surface.
Using submicron-sized particles is necessary for the purposes of targeting mitochondria. Mitochondria targeting polymeric particles with diameters equal to or smaller than approximately 200 nm have be found in the mitochondria of HeLa cells21. Here, the mean hydrodynamic diameter of the particles was approximately 200 nm. The mechanism of how polymeric particles can locate to mitochondria remains unclear. However, it is likely that the TPP group on the particle surface plays an important role in a unique endosomal escape pathway after cellular uptake43 and facilitates particle transport to the inside of mitochondria with its binding ability to the matrix of the negatively charged mitochondrial inner membrane44.
Whilst TPP is a desirable agent to include into particulate formulations designed to target mitochondria, excessive TPP accumulation in mitochondria is arguably toxic to cells45, 46. Examples of toxic effects include the inhibition of the mitochondrial electron transport chain and inducing mitochondrial proton leakage45. In order to minimize possible cytotoxicity of TPP cations, short linker chains of the TPP cation, (4-carboxybutyl) triphenylphosphonium bromide, were used in the studies presented here. Furthermore, the amount of TPP used was minimized by conjugating the TPP group onto the surface of PLGA particles as opposed to every single polymer chain. This was done by adding TPP-EDC-NHS in 0.1 M MES buffer solution into the formulation after the CIP were formed. Since the conjugation was conducted before the particles were washed and lyophilized, the loss in drug loading (CaMKIIN peptide) during formulation was minimized; which is an outstanding feature of this novel method.
TPP-CIP were more readily taken up by cardiomyocyte-like H9c2 cells than non-targeting CIP as indicated in Figure 6A. This result is consistent with previously published data16, 39, 46. Bielski et al. found that conjugating TPP onto poly(amidoamine) (PAMAM) dendrimer nanocarriers increased both cellular internalization and mitochondrial targeting in the human lung adenocarcinoma cell line, A54916. Guzman-Villaneuva et al. (2015) reported that TPP-liposomes had higher cellular uptake than liposomes without surface-conjugated TPP46.
CaMKII is a multifunctional signaling enzyme expressed in heart cells which can be activated by stimulating the β-adrenergic receptor47. To study the effect of CaMKII inhibition by TPP-CIP, CaMKII induction is necessary. This can be done by adding a β-adrenergic receptor agonist called isoprenaline (ISO) to differentiated H9c2 cells48, 49. It is known that CaMKII activity promotes mitochondrial-triggered cell death due to Ca2+ overload and excess ROS50. Since ISO treatment increases mitochondrial ROS production, the reduction in intracellular ROS production in cells pretreated with TPP-CIP indicates the ability of these particles to inhibit CaMKII. Cells pretreated with TPP-CIP was the only group which had intracellular ROS (pro-oxidants) levels significantly lower than cells treated with ISO alone, suggesting that TPP-CIP helped to protect cells against ISO induced intracellular ROS generation (Figure 6B).
In addition to intracellular ROS, mitochondrial ΔΨ of differentiated H9c2 cells was also measured. ΔΨ is the total transmembrane electrical potential or voltage gradient which is an indicator of the health of cells51, 52. TMRM is a fluorescent lipophilic cationic dye which can accumulate within mitochondria at levels that depend on the ΔΨ value. Since the inner membrane of polarized mitochondria have a higher negative charge than depolarized mitochondria, TMRM can accumulate more in polarized mitochondria53. During cellular stress, as when ISO is added, ΔΨ can be altered by the intracellular ionic charges such as Ca2+ 54 which result in ΔΨ collapse and mitochondria depolarization53. From the results shown here, TPP-CIP can protect cardiomyocyte-like cells from ISO-induced mitochondrial damage as seen from the reduction in intracellular ROS (pro-oxidants) (Figure 6B) and the high ΔΨ (Figure 7).
4. Conclusion
This study reports on the development of a new submicron sized particulate drug delivery system carrying a CaMKIIN peptide designed to protect cells against mitochondrial injury. The cellular uptake and mitochondrial targeting ability was achieved through the surface conjugation of PLGA-based carriers with a mitochondrial targeting molecule, TPP. The conjugation with TPP was confirmed using zeta potential measurements and an assay measuring fluorescamine reactivity. TPP-CIP protected cardiomyocyte-like cells from ISO-induced ROS production and decreased ΔΨ. TPP-CIP have the potential to be used in protection against ischemia/reperfusion injury in susceptible patients such as patients undergoing heart surgery.
Supplementary Material
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health (P30 CA086862) and the Lyle and Sharon Bighley Professorship. M. A. Joiner acknowledges support from the National Institutes of Health Research Project Grant Program (R01 NS084190), to Amy Lee and to Carver Collaborative grant (MAJ). A. Wongrakpanich acknowledges financial support from The Royal Thai Government Scholarship.
Abbreviations
- CaMKII
the calcium and calmodulin (Ca2+/CaM) -dependent protein kinase II (CaMKII) enzyme
- CaMKIIN
the calcium and calmodulin (Ca2+/CaM) -dependent protein kinase II (CaMKII) inhibitor
- CIP
CaMKIIN peptide-loaded particles
- CISol
CaMKIIN peptide in soluble form
- DHE
dihydroethidium
- ISO
isoprenaline
- mPTP
the mitochondrial permeability transition pore
- PLGA
poly(lactic-co-glycolic acid)
- TPP
triphenylphosphonium cation
- TPP-CIP
TPP functionalized CaMKIIN peptide loaded particles
- ΔΨ
mitochondrial membrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mendis S, Puska P, Norrving B, Organization WH, Federation WH, Organization WS. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization. Global Atlas on Cardiovascular Disease Prevention and Control. 2011 [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell S. Emergency management of acute myocardial infarction. British Journal of Clinical Pharmacology. 1999;48(3):284–298. doi: 10.1046/j.1365-2125.1999.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. The Journal of Clinical Investigation. 123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491(7423):269–73. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joiner ML, Koval OM. CaMKII and stress mix it up in mitochondria. Front Pharmacol. 2014;5:67. doi: 10.3389/fphar.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correll RN, Molkentin JD. CaMKII does it again: even the mitochondria cannot escape its influence. Circ Res. 2013;112(9):1208–11. doi: 10.1161/CIRCRESAHA.113.301263. [DOI] [PubMed] [Google Scholar]
- 8.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95(18):10890–5. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang BH, Mukherji S, Soderling TR. Calcium/calmodulin-dependent protein kinase II inhibitor protein: localization of isoforms in rat brain. Neuroscience. 2001;102(4):767–77. doi: 10.1016/s0306-4522(00)00520-0. [DOI] [PubMed] [Google Scholar]
- 10.Tao-Cheng JH, Yang Y, Bayer KU, Reese TS, Dosemeci A. Effects of CaMKII inhibitor tatCN21 on activity-dependent redistribution of CaMKII in hippocampal neurons. Neuroscience. 2013;244:188–96. doi: 10.1016/j.neuroscience.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–22. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D, L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125(3):193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Sah H, Thoma LA, Desu HR, Sah E, Wood GC. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. International Journal of Nanomedicine. 2013;8:747–765. doi: 10.2147/IJN.S40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomedicine. 2015;10:1001–18. doi: 10.2147/IJN.S56932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81(2):127–38. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- 16.Bielski ER, Zhong Q, Brown M, da Rocha SR. Effect of the Conjugation Density of Triphenylphosphonium Cation on the Mitochondrial Targeting of Poly(amidoamine) Dendrimers. Mol Pharm. 2015;12(8):3043–53. doi: 10.1021/acs.molpharmaceut.5b00320. [DOI] [PubMed] [Google Scholar]
- 17.Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263(3):709–16. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 18.Murphy MP. Targeting lipophilic cations to mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777(7–8):1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Han M, Vakili MR, Soleymani Abyaneh H, Molavi O, Lai R, Lavasanifar A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol Pharm. 2014;11(8):2640–9. doi: 10.1021/mp500038g. [DOI] [PubMed] [Google Scholar]
- 20.Wongrakpanich A, Geary SM, Joiner M-IA, Anderson ME, Salem AK. Mitochondria-targeting particles. Nanomedicine (London, England) 2014;9(16):2531–2543. doi: 10.2217/nnm.14.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A. 2012;109(40):16288–93. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69(6):1476–86. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 23.Bonavita F, Stefanelli C, Giordano E, Columbaro M, Facchini A, Bonafè F, Caldarera CM, Guarnieri C. H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: inhibition by PMA. FEBS Letters. 2003;536(1–3):85–91. doi: 10.1016/s0014-5793(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 24.Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochimica et biophysica acta. 2015;1853(2):276–284. doi: 10.1016/j.bbamcr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y-W, Chou H-C, Lin S-T, Chen Y-H, Chang Y-J, Chen L, Chan H-L. Cardioprotective Effects of Quercetin in Cardiomyocyte under Ischemia/Reperfusion Injury. Evidence-Based Complementary and Alternative Medicine. 2013;2013:16. doi: 10.1155/2013/364519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng YW, Buller CL, Charpie JR. Impact of N-Acetylcysteine on Neonatal Cardiomyocyte Ischemia-Reperfusion Injury. Pediatr Res. 2011;70(1):61–66. doi: 10.1203/PDR.0b013e31821b1a92. [DOI] [PubMed] [Google Scholar]
- 27.Ronkainen JJ, Hänninen SL, Korhonen T, Koivumäki JT, Skoumal R, Rautio S, Ronkainen V-P, Tavi P. Ca2+–calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel α1C-subunit gene (Cacna1c) by DREAM translocation. The Journal of Physiology. 2011;589(11):2669–2686. doi: 10.1113/jphysiol.2010.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Cheng G, Jin R, Chen L, Chen X, Davani A, Samanta A, Girgis M, Choksi K, Yang Y, Vincent R, Dawn B. CAMKII IS CRITICAL FOR THE UPREGULATION OF STAT3 SIGNALING IN PATHOGENESIS OF CARDIOMYOCYTE HYPERTROPHY. Journal of the American College of Cardiology. 2015;65(10_S) [Google Scholar]
- 29.Hoch B, Haase H, Schulze W, Hagemann D, Morano I, Krause EG, Karczewski P. Differentiation-dependent expression of cardiac delta-CaMKII isoforms. J Cell Biochem. 1998;68(2):259–68. [PubMed] [Google Scholar]
- 30.Cipolletta E, Rusciano MR, Maione AS, Santulli G, Sorriento D, Del Giudice C, Ciccarelli M, Franco A, Crola C, Campiglia P, Sala M, Gomez-Monterrey I, De Luca N, Trimarco B, Iaccarino G, Illario M. Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy. PLoS ONE. 2015;10(6):e0130477. doi: 10.1371/journal.pone.0130477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comelli M, Domenis R, Bisetto E, Contin M, Marchini M, Ortolani F, Tomasetig L, Mavelli I. Cardiac differentiation promotes mitochondria development and ameliorates oxidative capacity in H9c2 cardiomyoblasts. Mitochondrion. 2011;11(2):315–26. doi: 10.1016/j.mito.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Song KC, Lee HS, Choung IY, Cho KI, Ahn Y, Choi EJ. The effect of type of organic phase solvents on the particle size of poly(d, l-lactide-co-glycolide) nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2006;276(1–3):162–167. [Google Scholar]
- 33.Gross B, Wongrakpanich A, Francis M, Salem A, Norian L. A Therapeutic Microparticle-Based Tumor Lysate Vaccine Reduces Spontaneous Metastases in Murine Breast Cancer. The AAPS Journal. 2014;16(6):1194–1203. doi: 10.1208/s12248-014-9662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng FT, Ma GH, Qiu W, Su ZG. W/O/W double emulsion technique using ethyl acetate as organic solvent: effects of its diffusion rate on the characteristics of microparticles. J Control Release. 2003;91(3):407–16. doi: 10.1016/s0168-3659(03)00273-6. [DOI] [PubMed] [Google Scholar]
- 35.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. Aaps j. 2013;15(1):85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178(4063):871–2. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 37.Branco AF, Sampaio SF, Moreira AC, Holy J, Wallace KB, Baldeiras I, Oliveira PJ, Sardao VA. Differentiation-dependent doxorubicin toxicity on H9c2 cardiomyoblasts. Cardiovasc Toxicol. 2012;12(4):326–40. doi: 10.1007/s12012-012-9177-8. [DOI] [PubMed] [Google Scholar]
- 38.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523–35. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty A, Jana NR. Design and Synthesis of Triphenylphosphonium Functionalized Nanoparticle Probe for Mitochondria Targeting and Imaging. The Journal of Physical Chemistry C. 2015;119(5):2888–2895. [Google Scholar]
- 40.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98(2):367–81. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 41.Branco AF, Pereira SP, Gonzalez S, Gusev O, Rizvanov AA, Oliveira PJ. Gene Expression Profiling of H9c2 Myoblast Differentiation towards a Cardiac-Like Phenotype. PLoS ONE. 2015;10(6):e0129303. doi: 10.1371/journal.pone.0129303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Branco AF, Pereira SL, Moreira AC, Holy J, Sardao VA, Oliveira PJ. Isoproterenol cytotoxicity is dependent on the differentiation state of the cardiomyoblast H9c2 cell line. Cardiovasc Toxicol. 2011;11(3):191–203. doi: 10.1007/s12012-011-9111-5. [DOI] [PubMed] [Google Scholar]
- 43.Pathak RK, Kolishetti N, Dhar S. Targeted Nanoparticles in Mitochondrial Medicine. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2015;7(3):315–329. doi: 10.1002/wnan.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777(7–8):1028–31. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 45.Trnka J, Elkalaf M, Andel M. Lipophilic triphenylphosphonium cations inhibit mitochondrial electron transport chain and induce mitochondrial proton leak. PLoS One. 2015;10(4):e0121837. doi: 10.1371/journal.pone.0121837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzman-Villanueva D, Mendiola MR, Nguyen HX, Weissig V. Influence of Triphenylphosphonium (TPP) Cation Hydrophobization with Phospholipids on Cellular Toxicity and Mitochondrial Selectivity. SOJ Pharm Pharm Sci. 2015;2(1):1–9. [Google Scholar]
- 47.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48(2):322–30. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curran J, Tang L, Roof SR, Velmurugan S, Millard A, Shonts S, Wang H, Santiago D, Ahmad U, Perryman M, Bers DM, Mohler PJ, Ziolo MT, Shannon TR. Nitric oxide-dependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS One. 2014;9(2):e87495. doi: 10.1371/journal.pone.0087495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popolo A, Pecoraro M, Pinto A. Effect of Adenosine on Isoproerenol-induced Hypertrophy In Vitro. A Preliminary Study. Pharmacologyonline. 2014;1:121–126. [Google Scholar]
- 50.Joiner M-IA, Koval OM. CaMKII and stress mix it up in mitochondria. Frontiers in Pharmacology. 2014;5:67. doi: 10.3389/fphar.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solaini G, Sgarbi G, Lenaz G, Baracca A. Evaluating mitochondrial membrane potential in cells. Biosci Rep. 2007;27(1–3):11–21. doi: 10.1007/s10540-007-9033-4. [DOI] [PubMed] [Google Scholar]
- 52.Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430. doi: 10.1038/cddis.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


