Figure 1.
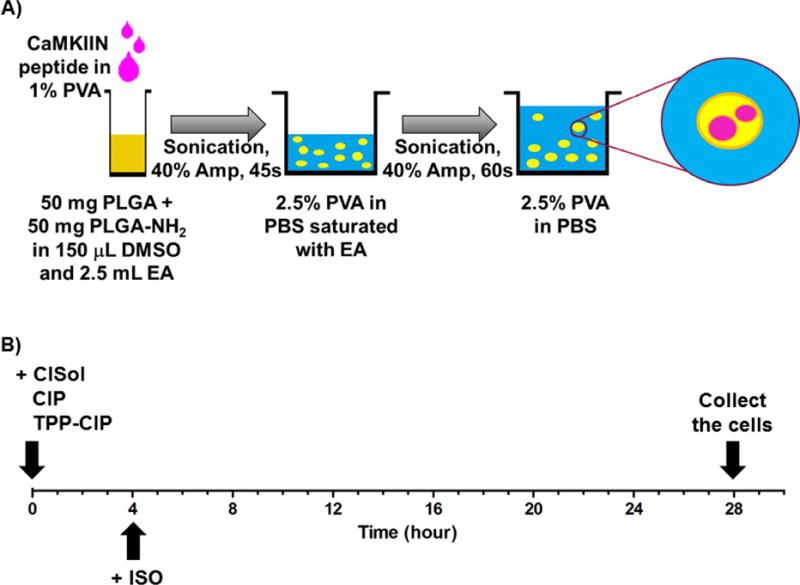
(A) Fabrication of CaMKIIN-loaded particles (CIP): schematic of the protocol for loading CaMKIIN peptides into PLGA particles (see method for further details). PLGA, poly(lactic-co-glycolic-acid); PLGA-NH2, amine endcapped poly(lactic-co-glycolic-acid); PVA, polyvinyl alcohol; EA, ethyl acetate; Amp, amplitude.
(B) Timeline of in vitro experiments with differentiated H9c2 cells: cells were treated with different formulations of CaMKIIN peptide at t = 0, incubated with ISO at t = 4 and collected at t = 28. CaMKIIN peptide solution (CISol); CaMKIIN loaded particles (CIP); TPP conjugated CaMKIIN loaded particles (TPP-CIP); Isoprenaline (ISO).
