Fig. 10.
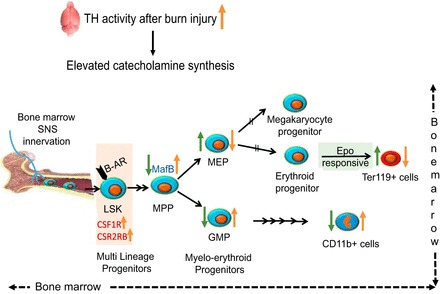
Schematic diagram representing the alterations in myelo-erythroid commitment pattern leading to red blood cell deficits after burn injury in the mouse model (trend shown in orange arrows). 15% TBSA in mouse scald burn model stimulates increased catecholamine synthesis as shown by increased TH levels in the brain, one of the most active organs of sympathetic nerve system. Bone marrow is highly innervated; LSKs in the bone marrow that express β-adrenergic receptor (β-AR) respond to increased catecholamines. Increased gene expression of CSF1R and CSF2RB in LSKs after burn likely gears them toward myeloid lineage. The MPPs through increased expression of MafB after burn injury differentiate away from erythroid progenitors (MEPs) and toward myeloid progenitors (GMPs). Administration of a β-AR blocker (PR) (trend shown in green arrows) reverses these effects by decreasing MafB expression, decreasing GMPs, and increasing MEP production. In line with progenitors, Ter119+ cells were increased after PR treatment, whereas mature myeloid cells represented by CD11b+ marker decreased. As MEPs are common progenitors to megakaryocytes and erythrocytes, their erythroid potential was verified by their responsiveness to Epo after PR treatment in burn mice.
