Figure 1.
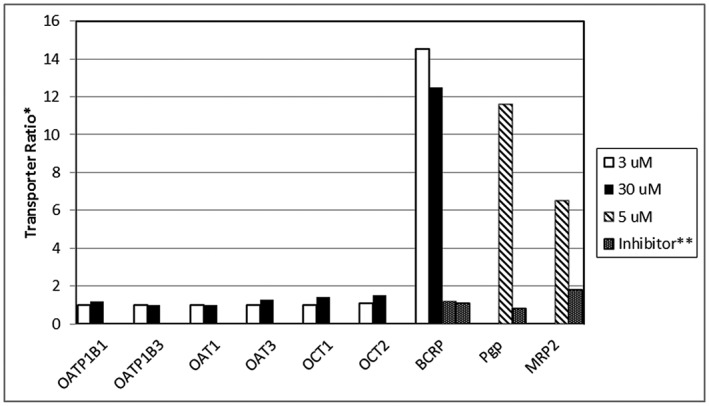
In vitro evaluation of canagliflozin as a substrate of human uptake and efflux transporters. *Transporter ratio for the efflux transporters (P‐glycoprotein [Pgp], multidrug resistance‐associated protein‐2 [MRP2], breast cancer resistant protein [BCRP]) is based on the permeability of canagliflozin from A‐B/B‐A. For the uptake transporters (organic anion transporter polypeptide isoforms OAT1B1, OATP1B3, organic anion transporters OAT1 and OAT3, and organic cationic transporters OCT1 and OCT2) transporter ratio is based on the uptake of canagliflozin in transporter containing system to control (water injected oocyte system or parental HEK293 line). **Ratio in presence of specific transport inhibitor (1 μmol l–1 KO143 for BCRP, 100 μmol l–1 cyclosporine for Pgp or 100 μmol l–1 cisplatin for MRP2)
