Fig. 1.
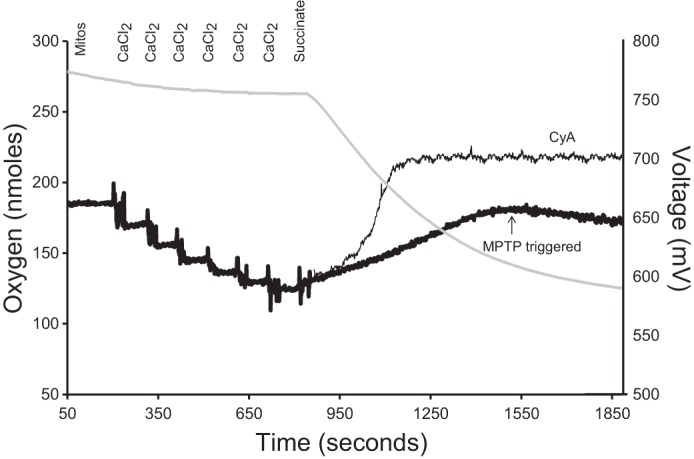
A representative model for estimating calcium-loading capacity in isolated brain mitochondria. Mitochondrial calcium (black line traces) and oxygen consumption (gray line trace) were recorded simultaneously using a Clark-type oxygen electrode equipped with a CaCl2-selective electrode. The calcium electrode measures extramitochondrial calcium as an electric potential between the assay media and the electrode filling solution, which contains 10 mM CaCl2. In 1 ml of respiration buffer at 25°C, 0.5 mg mitochondria, rotenone (2 mM, dissolved in ethanol), and oligomycin (1 mg/ml, dissolved in ethanol) were added. Once a steady-state reading was established, sequential additions of CaCl2 were made to calibrate the calcium electrode (20 μM, 20 μM, 40 μM, 80 μM, and 100 μM, total 260 μM). Additions of CaCl2 are observed as a decrease in potential at the calcium electrode. When the mitochondria are energized (i.e., succinate addition), the calcium is sequestered in the mitochondria, which decreases the extramitochondrial CaCl2 concentration, and is measured as an increase in electrical potential by the electrode. Calcium-loading capacity was estimated as the maximum amount of CaCl2 sequestered by mitochondria before CaCl2 loading halted (i.e., the mitochondrial permeability transition pore (MPTP) was triggered, indicated by thick, black line). Assay was repeated with the addition of 1 μM cyclosporin A (CyA, a specific inhibitor of MPTP) to confirm opening of MPTP through the blockage of CaCl2 release back into the medium (indicated by thin, black line).
