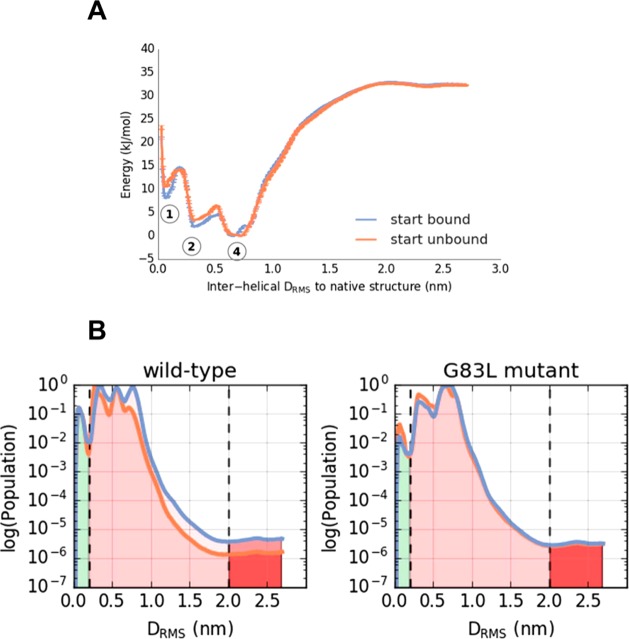
Figure 6.
Effects of a “disruptive” mutation on the dimerization of the TM helix of GpA. (A) PMF for dimerization of a mutant (G83L) of GpA (compare with the wild type PMF in Figure 4A). The three energy minima labeled correspond to the structures in Figure 4C. Two initial conditions were used, which had either all replicas in the native configuration (“bound”, blue), or all replicas initially dissociated (“unbound”, orange). (B) Comparison of the population distributions derived from the wild-type and G83L GpA PMFs. From these one may estimate a ΔΔG of 0 or 4 kJ/mol (with unbound state defined at 2.0 nm or 0.2 DRMS respectively) for destabilization of the native dimer by the G83L mutation.