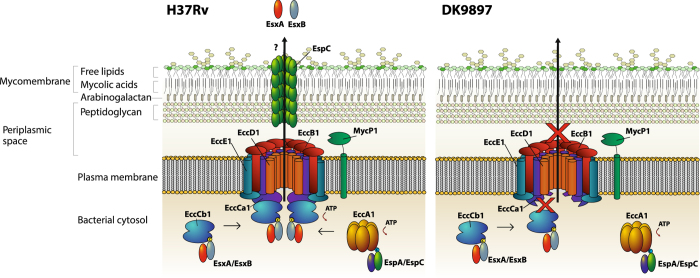
Figure 6. Model of the functional ESX-1 secretion apparatus and the effect of the modified eccCa1 (Rv3870) in M.tb DK9897.
(a) The core structure of the ESX-1 system is formed by the conserved components EccB1, EccCa1, EccCb1, EccD1 and EccE1, each possessing transmembrane region(s) spanning the mycobacterial plasma membrane64. The EccC is an ATP-driven translocase consisting of two subunits, EccCa1 and EccCb1, which are assembled once EccCb1 binds its target substrate, in this case, the EsxA/EsxB heterodimer, where EccCb1 interacts with the carboxy-terminal signal sequence of EsxB (marked as C in the figure)65,66. EsxB functions as a chaperone for EsxA secretion, which is a major ESX-1 virulence factor21. The secretion of EsxA/EsxB is co-dependent with the secretion of EspC/EspA and the C-terminus of EspC targets for the interaction with the cytosolic ATPase EccA167,68. EspC polymerizes during secretion, indicating that EccA1 and EspA might function as cytosolic chaperones69. The polymerization of EspC results in the formation of a surface-exposed filamentous structure that spans the entire cell envelope69, possibly serving as a channel responsible for transporting ESX-1 substrates. (b) The truncated EccCa1 of M.tb DK9897 is unable to bind the EccCb1/EsxA/EsxB complex and is thus unable to secrete the EsxA/EsxB heterodimer. The secretion of EsxA/EsxB and EspA/EspC are mutually co-dependent67, meaning that the secretion of EspC is presumably also disrupted in M.tb DK9897.