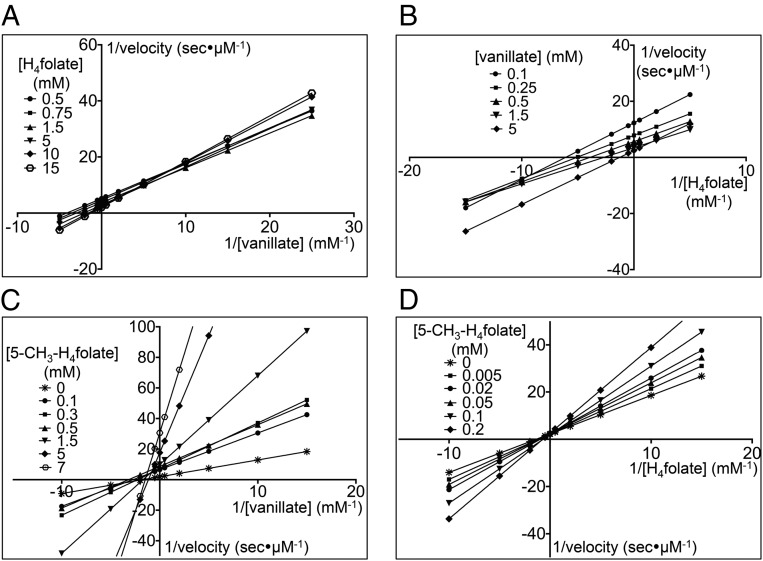
Fig. 2.
LigM uses an ordered, sequential kinetic mechanism. The steady-state kinetics for LigM O-demethylation of vanillate was determined by varying the concentration of one substrate while the concentration of the other substrate was fixed at different concentrations. (A and B) For visualization purposes, data were plotted as Lineweaver–Burk plots with 1/velocity (s/μM) vs. 1/[vanillate] (mM) at different concentrations of H4folate (in mM) (A), and 1/velocity (s/μM) vs.1/[H4folate] (mM) at different concentrations of vanillate (in mM) (B). Both families of plots, with respect to vanillate and to H4folate, show an intersecting pattern, suggesting a sequential mechanism for LigM. (C and D) Product inhibition studies using 5-methyl-H4folate (5-CH3-H4folate) were performed to distinguish between ordered and random sequential mechanisms. To identify the resulting inhibition pattern, data were transformed into Lineweaver–Burk plots with 1/velocity (in s/μM) vs. 1/[vanillate] (in mM) and the concentration of 5-CH3-H4folate ranging from 0–7 mM (C) and 1/velocity (in s/μM) vs. 1/[H4folate] (in mM) with the concentration of 5-CH3-H4folate ranging from 0–0.2 mM (D). In respect to vanillate, 5-CH3-H4folate is a noncompetitive inhibitor (C); however, 5-CH3-H4folate acts as a competitive inhibitor with respect to H4folate (D). These data support an ordered, sequential mechanism for LigM. For determination of kinetic parameters (Table 1), velocity vs. substrate concentration curves (SI Appendix, Fig. S1) were fit globally with the appropriate nonlinear least-squares regression rate equations.