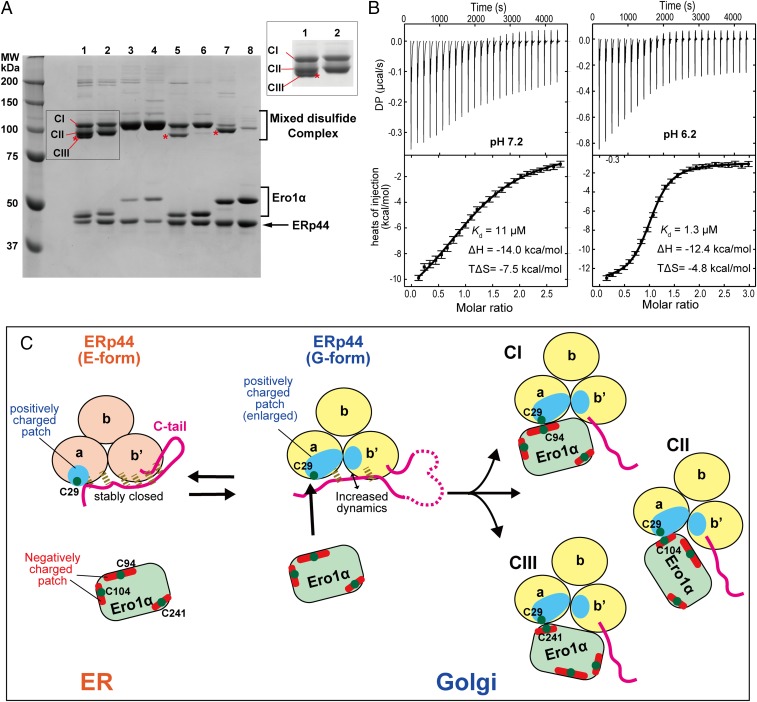
Fig. 5.
Modes of interaction between ERp44 and Ero1α. (A) Disulfide-bond formation of ERp44 with WT and cysteine mutants of Ero1α. ERp44 was mixed with each of the indicated Ero1α mutants at an equal molar ratio (5 µM). The mixture was treated with 1 mM NEM and then subjected to nonreducing SDS/PAGE. Asterisks indicate the complexes involving Cys208 or Cys241 of Ero1α. Lane 1, wild type; lane 2, WTSS (C208S/C241S); lane 3, HA (C104A/C131A); lane 4, HASS (C104A/C131A/C208S/C241S); lane 5, IA (C94A/C104A); lane 6, IASS (C94A/C104A/C208S/C241S); lane 7, Cysless (C94A/C99A/C104A/C131A); lane 8, CyslessSS (C94A/C99A/C104A/C131A/C208S/C241S). (B) ITC raw data (Upper) and binding isotherm data (Lower) for titration of Ero1α HA into ERp44 at pH 7.2 (Left) or 6.2 (Right). DP indicates a measured differential power between the reference and sample cells. Error bars represent errors estimated by a program NITPIC (49) for each integrated heat. (C) Proposed working model for the pH-dependent regulation of ERp44. Green dots represent cysteine residues that could be involved in the covalent complex. Dashed lines represent hydrogen bonds formed between the C tail and the main body of ERp44. CI, CII, and CIII denote the three types of mixed disulfide complexes between ERp44 and Ero1α suggested by experiments shown in A.